Search results
Search for "N-heterocyclic carbene" in Full Text gives 124 result(s) in Beilstein Journal of Organic Chemistry.
A laterally-fused N-heterocyclic carbene framework from polysubstituted aminoimidazo[5,1-b]oxazol-6-ium salts
- Andrew D. Gillie,
- Matthew G. Wakeling,
- Bethan L. Greene,
- Louise Male and
- Paul W. Davies
Beilstein J. Org. Chem. 2024, 20, 621–627, doi:10.3762/bjoc.20.54

Graphical Abstract

Figure 1: Laterally fused NHC motifs.

Scheme 1: Synthetic studies into the formation of a 3-aminoimdazo[5,1-b]oxazol-6-ium motif based on a gold-ca...

Scheme 2: The synthesis of AImOxAu(I)Cl, AImOxCu(I)Cl, and AImOxIr(CO)2Cl complexes from 6a. The single cryst...

Scheme 3: Use of AImOxAuCl 13 in catalysis. aYields are calculated from the 1H NMR spectra against an interna...
Mechanisms for radical reactions initiating from N-hydroxyphthalimide esters
- Carlos R. Azpilcueta-Nicolas and
- Jean-Philip Lumb
Beilstein J. Org. Chem. 2024, 20, 346–378, doi:10.3762/bjoc.20.35
- , accompanied by the formation of cyano-B(pin) 142. Importantly, in the absence of ZnCl2, intermediate 140 could be detected by HRMS and 11B NMR, highlighting the pivotal role of ZnCl2 in promoting rearomatization. N-Heterocyclic carbene (NHC) catalysis In 2019, Nagao and Ohmiya reported the decarboxylative
- overview of the diverse mechanisms that have been proposed for radical based transformations initiating from NHPI esters. The discussion is organized into four sections: (i) mechanisms under photochemical conditions, (ii) initiation by metal catalysis and stoichiometric reductants, (iii) N-heterocyclic
- carbene (NHC)-catalyzed radical relay, and (iv) mechanisms under electrochemical activation. By discussing selected literature examples, we illustrate how the activation mode of NHPI esters, and the reactivity of the resulting radical species, can vary depending upon the choice of catalytic or

Graphical Abstract

Scheme 1: Comparison between Barton and NHPI ester radical precursors.

Scheme 2: Overview of the mechanisms and activation modes involved in radical generation from RAEs.

Scheme 3: Common mechanisms in photocatalysis.

Scheme 4: A) Giese-type radical addition of NHPI esters mediated by a reductive quenching photocatalytic cycl...

Scheme 5: A) Minisci-type radical addition of NHPI esters. B) Reaction mechanism involving an “off-cycle” red...

Scheme 6: Activation of NHPI esters through hydrogen-bonding in an oxidative quenching photocatalytic cycle.

Scheme 7: SET activation of RAE facilitated by a Lewis acid catalyst.

Scheme 8: PCET activation of NHPI esters in the context of a radical-redox annulation.

Scheme 9: Activation enabled by a strong excited-state reductant catalyst and its application in the dearomat...

Scheme 10: Proposed formation of an intramolecular charge-transfer complex in the synthesis of (spiro)anellate...

Scheme 11: Formation of a charge-transfer complex between enamides and NHPI esters enabled by a chiral phospha...

Scheme 12: Activation of NHPI ester through the formation of photoactive EDA-complexes.

Scheme 13: A) EDA complex-mediated radical hydroalkylation reactions of NHPI esters. B) Proposed mechanism for...

Scheme 14: Proposed radical chain mechanism initiated by EDA-complex formation.

Scheme 15: A) Photoinduced decarboxylative borylation. B) Proposed radical chain mechanism.

Scheme 16: A) Activation of NHPI esters mediated by PPh3/NaI. B) Proposed catalytic cycle involving EDA-comple...

Scheme 17: A) Radical generation facilitated by EDA complex formation between PTH1 catalyst and NHPI esters. B...

Scheme 18: Proposed catalytic cycle for the difunctionalization of styrenes.

Scheme 19: Formation of a charge-transfer complex between NHPI esters and Cs2CO3 enables decarboxylative amina...

Scheme 20: 3-Acetoxyquinuclidine as catalytic donor in the activation of TCNHPI esters.

Scheme 21: A) Photoinduced Cu-catalyzed decarboxylative amination. B) Proposed catalytic cycle. C) Radical clo...

Scheme 22: A) Photoinduced Pd-catalyzed aminoalkylation of 1,4-dienes. B) Proposed catalytic cycle.

Scheme 23: A) TM-catalyzed decarboxylative coupling of NHPI esters and organometallic reagents. B) Representat...

Scheme 24: Synthetic applications of the TM-catalyzed decarboxylative coupling of NHPI esters and organometall...

Scheme 25: A) Ni-catalyzed cross-electrophile coupling of NHPI esters. B) Representative catalytic cycle.

Scheme 26: A) Synthetic applications of decarboxylative cross-electrophile couplings. B) Decarboxylative aryla...

Scheme 27: A) Activation of tetrachlorophthalimide redox-active esters enabled by a low-valency Bi complex. B)...

Scheme 28: Activation of NHPI esters mediated by Zn0 applied in a Z-selective alkenylation reaction.

Scheme 29: A) Activation of NHPI esters enabled by a pyridine-boryl radical species applied to the decarboxyla...

Scheme 30: A) Decarboxylative coupling of RAE and aldehydes enabled by NHC-catalyzed radical relay. B) Propose...

Scheme 31: A) Decarboxylative C(sp3)–heteroatom coupling reaction of NHPI esters under NHC catalysis B) The NH...

Scheme 32: A) Electrochemical Giese-type radical addition of NHPI esters. B) Reaction mechanism.

Scheme 33: Electrochemical Minisci-type radical addition of NHPI-esters.

Scheme 34: Ni-electrocatalytic cross-electrophile coupling of NHPI esters with aryl iodides.

Scheme 35: A) Decarboxylative arylation of NHPI esters under Ag-Ni electrocatalysis B) Formation of AgNP on th...

Scheme 36: Synthetic applications of decarboxylative couplings of NHPI esters under Ni-electrocatalysis.

Scheme 37: Examples of natural product syntheses in which RAEs were used in key C–C bond forming reactions.
Active-metal template clipping synthesis of novel [2]rotaxanes
- Cătălin C. Anghel,
- Teodor A. Cucuiet,
- Niculina D. Hădade and
- Ion Grosu
Beilstein J. Org. Chem. 2023, 19, 1776–1784, doi:10.3762/bjoc.19.130

- molecule which catalyzes the macrocyclization reaction around the axle (Figure 1b). Results and Discussion In order to access the target [2]rotaxanes we made use of the CuAAC reaction, performed in the presence of a copper(I) N-heterocyclic carbene, a very stable and efficient class of catalysts used in
- active-metal template and clipping methods to yield the target interlocked molecules via [1 + 1] or intramolecular macrocyclizations, realized through CuAAC reactions catalyzed by axle-copper(I) N-heterocyclic carbene complexes. The [2]rotaxane obtained by [1 + 1] clipping (R1) could be only observed by

Graphical Abstract

Figure 1: a. Active-metal template (reported in the literature) and b. active-metal template clipping (used i...

Figure 2: Macrocyclic components of the [2]rotaxanes.

Scheme 1: Synthesis of the key intermediates 6 and 8 and of the reference macrocycle M1.

Scheme 2: Synthesis of [2]rotaxanes R1 and R2.

Figure 3: Top: HRESI(+)-MS spectrum of the rotaxane R1 (left) and R2 (right) [experimental (top) and calculat...
Application of N-heterocyclic carbene–Cu(I) complexes as catalysts in organic synthesis: a review
- Nosheen Beig,
- Varsha Goyal and
- Raj K. Bansal
Beilstein J. Org. Chem. 2023, 19, 1408–1442, doi:10.3762/bjoc.19.102
- diaminocarbene complex 32 and diamidocarbene (DAC) complex 33. The mononuclear N-heterocyclic carbene (NHC)–copper alkoxide complexes [(6-NHC)CuOt-Bu] (6-NHC = 6-MesDAC (30), 6-Mes (31)) were prepared by the addition of free carbenes to the tetrameric tert-butoxide precursor [Cu(Ot-Bu)]4, or by protonolysis of

Graphical Abstract

Scheme 1: In situ generation of imidazolylidene carbene.

Scheme 2: Hg(II) complex of NHC.

Scheme 3: Isolable and bottlable carbene reported by Arduengo [3].

Scheme 4: First air-stable carbene synthesized by Arduengo in 1992 [5].

Figure 1: General structure of an NHC.

Figure 2: Stabilization of an NHC by donation of the lone pair electrons into the vacant p-orbital (LUMO) at ...

Figure 3: Abnormal NHC reported by Bertrand [8,9].

Figure 4: Cu(d) orbital to σ*C-N(NHC) interactions in NHC–CuX complexes computed at the B3LYP/def2-SVP level ...

Figure 5: Molecular orbital contributions to the NHC–metal bond.

Scheme 5: Synthesis of NHC–Cu(I) complexes by deprotonation of NHC precursors with a base.

Scheme 6: Synthesis of [NHC–CuX] complexes.

Scheme 7: Synthesis of [(ICy)CuX] and [(It-Bu)CuX] complexes.

Scheme 8: Synthesis of iodido-bridged copper–NHC complexes by deprotonation of benzimidazolium salts reported...

Scheme 9: Synthesis of copper complexes by deprotonation of triazolium salts.

Scheme 10: Synthesis of thiazolylidene–Cu(I) complex by deprotonation with KOt-Bu.

Scheme 11: Preparation of NHC–Cu(I) complexes.

Scheme 12: Synthesis of methylmalonic acid-derived anionic [(26a,b)CuCl]Li(THF)2 and zwitterionic (28) heterol...

Scheme 13: Synthesis of diaminocarbene and diamidocarbene (DAC)–Cu(I) complexes.

Scheme 14: Synthesis of the cationic (NHC)2Cu(I) complex 39 from benzimidazolium salts 38 with tetrakis(aceton...

Scheme 15: Synthesis of NHC and ADC (acyclic diamino carbenes) Cu(I) hexamethyldisilazide complexes reported b...

Scheme 16: Synthesis of NHC–copper(I) complexes using an acetylacetonate-functionalized imidazolium zwitterion...

Scheme 17: Synthesis of NHC–Cu(I) complexes through deprotonation of azolium salts with Cu2O.

Scheme 18: Synthesis of NHC–CuBr complex through deprotonation with Cu2O reported by Kolychev [31].

Scheme 19: Synthesis of chiral NHC–CuBr complexes from phenoxyimine-imidazolium salts reported by Douthwaite a...

Scheme 20: Preparation of linear neutral NHC–CuCl complexes through the use of Cu2O. For abbreviations, please...

Scheme 21: Synthesis of abnormal-NHC–copper(I) complexes by Bertrand, Cazin and co-workers [35].

Scheme 22: Microwave-assisted synthesis of thiazolylidene/benzothiazolylidene–CuBr complexes by Bansal and co-...

Scheme 23: Synthesis of NHC–CuX complexes through transmetallation.

Scheme 24: Preparation of six- or seven-membered NHC–Cu(I) complexes through transmetalation from Ag(I) comple...

Scheme 25: Synthesis of 1,2,3-triazolylidene–CuCl complexes through transmetallation of Ag(I) complexes genera...

Scheme 26: Synthesis of NHC–copper complexes having both Cu(I) and Cu(II) units through transmetalation report...

Scheme 27: Synthesis of new [(IPr(CH2)3Si(OiPr)3)CuX] complexes and anchoring on MCM-41.

Scheme 28: Synthesis of bis(trimethylsilyl)phosphide–Cu(I)–NHC complexes through ligand displacement.

Scheme 29: Synthesis of silyl- and stannyl [(NHC)Cu−ER3] complexes.

Scheme 30: Synthesis of amido-, phenolato-, thiophenolato–Cu(NHC) complexes.

Scheme 31: Synthesis of first isolable NHC–Cu–difluoromethyl complexes reported by Sanford et al. [44].

Scheme 32: Synthesis of NHC–Cu(I)–bifluoride complexes reported by Riant, Leyssens and co-workers [45].

Scheme 33: Conjugate addition of Et2Zn to enones catalyzed by an NHC–Cu(I) complex reported by Woodward in 200...

Scheme 34: Hydrosilylation of a carbonyl group.

Scheme 35: NHC–Cu(I)-catalyzed hydrosilylation of ketones reported by Nolan et al. [48,49].

Scheme 36: Application of chiral NHC–CuCl complex 104 for the enantioselective hydrosilylation of ketones.

Scheme 37: Hydrosilylation reactions catalyzed by NHC–Cu(Ot-Bu) complexes.

Scheme 38: NHC–CuCl catalyzed carbonylative silylation of alkyl halides.

Scheme 39: Nucleophilic conjugate addition to an activated C=C bond.

Figure 6: Molecular electrostatic potential maps (MESP) of two NHC–CuX complexes computed at the B3LYP/def2-S...

Scheme 40: Conjugate addition of Grignard reagents to 3-alkyl-substituted cyclohexenones catalyzed by a chiral...

Scheme 41: NHC–copper complex-catalyzed conjugate addition of Grignard reagent to 3-substituted hexenone repor...

Scheme 42: Conjugate addition or organoaluminum reagents to β-substituted cyclic enones.

Scheme 43: Conjugate addition of boronates to acyclic α,β-unsaturated carboxylic esters, ketones, and thioeste...

Scheme 44: NHC–Cu(I)-catalyzed hydroboration of an allene reported by Hoveyda [63].

Scheme 45: Conjugate addition of Et2Zn to cyclohexenone catalyzed by NHC–Cu(I) complex derived from benzimidaz...

Scheme 46: Asymmetric conjugate addition of diethylzinc to 3-nonen-2-one catalyzed by NHC–Cu complexes derived...

Scheme 47: General scheme of a [3 + 2] cycloaddition reaction.

Scheme 48: [3 + 2] Cycloaddition of azides with alkynes catalyzed by NHC–Cu(I) complexes reported by Diez-Gonz...

Scheme 49: Application of NHC–CuCl/N-donor combination to catalyze the [3 + 2] cycloaddition of benzyl azide w...

Scheme 50: [3 + 2] Cycloaddition of azides with acetylenes catalyzed by bis(NHC)–Cu complex 131 and mixed NHC–...

Figure 7: NHC–CuCl complex 133 as catalyst for the [3 + 2] cycloaddition of alkynes with azides at room tempe...

Scheme 51: [3 + 2] Cycloaddition of a bulky azide with an alkynylpyridine using [(NHC)Cu(μ-I)2Cu(NHC)] copper ...

Scheme 52: [3 + 2] Cycloaddition of benzyl azide with phenylacetylene under homogeneous and heterogeneous cata...

Scheme 53: [3 + 2] Cycloaddition of benzyl azide with acetylenes catalyzed by bisthiazolylidene dicopper(I) co...

Figure 8: Copper (I)–NHC linear coordination polymer 137 and its conversion into tetranuclear (138) and dinuc...

Scheme 54: An A3 reaction.

Scheme 55: Synthesis of SiO2-immobilized NHC–Cu(I) catalyst 141 and its application in the A3-coupling reactio...

Scheme 56: Preparation of dual-purpose Ru@SiO2–[(NHC)CuCl] catalyst system 142 developed by Bordet, Leitner an...

Scheme 57: Application of the catalyst system Ru@SiO2–[Cu(NHC)] 142 to the one-pot tandem A3 reaction and hydr...

Scheme 58: A3 reaction of phenylacetylene with secondary amines and aldehydes catalyzed by benzothiazolylidene...

Figure 9: Kohn–Sham HOMOs of phenylacetylene and NHC–Cu(I)–phenylacetylene complex computed at the B3LYP/def2...

Figure 10: Energies of the FMOs of phenylacetylene, iminium ion, and NHC–Cu(I)–phenylacetylene complex compute...

Scheme 59: NHC–Cu(I) catalyzed diboration of ketones 147 by reacting with bis(pinacolato)diboron (148) reporte...

Scheme 60: Protoboration of terminal allenes catalyzed by NHC–Cu(I) complexes reported by Hoveyda and co-worke...

Scheme 61: NHC–CuCl-catalyzed borylation of α-alkoxyallenes to give 2-boryl-1,3-butadienes.

Scheme 62: Regioselective hydroborylation of propargylic alcohols and ethers catalyzed by NHC–CuCl complexes 1...

Scheme 63: NHC–CuOt-Bu-catalyzed semihydrogenation and hydroborylation of alkynes.

Scheme 64: Enantioselective NHC–Cu(I)-catalyzed hydroborations of 1,1-disubstituted aryl olefins reported by H...

Scheme 65: Enantioselective NHC–Cu(I)-catalyzed hydroboration of exocyclic 1,1-disubstituted alkenes reported ...

Scheme 66: Markovnikov-selective NHC–CuOH-catalyzed hydroboration of alkenes and alkynes reported by Jones et ...

Scheme 67: Dehydrogenative borylation and silylation of styrenes catalyzed by NHC–CuOt-Bu complexes developed ...

Scheme 68: N–H/C(sp2)–H carboxylation catalyzed by NHC–CuOH complexes.

Scheme 69: C–H Carboxylation of benzoxazole and benzothiazole derivatives with CO2 using a 1,2,3-triazol-5-yli...

Scheme 70: Use of Cu(I) complex derived from diethylene glycol-functionalized imidazo[1,5,a] pyridin-3-ylidene...

Scheme 71: Allylation and alkenylation of polyfluoroarenes and heteroarenes catalyzed by NHC–Cu(I) complexes r...

Scheme 72: Enantioselective C(sp2)–H allylation of (benz)oxazoles and benzothiazoles with γ,γ-disubstituted pr...

Scheme 73: C(sp2)–H arylation of arenes catalyzed by dual NHC–Cu/NHC–Pd catalytic system.

Scheme 74: C(sp2)–H Amidation of (hetero)arenes with N-chlorocarbamates/N-chloro-N-sodiocarbamates catalyzed b...

Scheme 75: NHC–CuI catalyzed thiolation of benzothiazoles and benzoxazoles.
Pyridine C(sp2)–H bond functionalization under transition-metal and rare earth metal catalysis
- Haritha Sindhe,
- Malladi Mounika Reddy,
- Karthikeyan Rajkumar,
- Akshay Kamble,
- Amardeep Singh,
- Anand Kumar and
- Satyasheel Sharma
Beilstein J. Org. Chem. 2023, 19, 820–863, doi:10.3762/bjoc.19.62
- with a bulky N-heterocyclic carbene ligand and (2,6-t-Bu2-4-Me-C6H2O)2AlMe (MAD) as the Lewis acid which allowed the direct C-4 alkylation of pyridines 1 (Scheme 12a). With the optimized reaction conditions in hand the group also screened the alkene and pyridine substrate scope which resulted C4
- oxidized by Cu(II) to complete the catalytic cycle. Next, considering the role of N-heterocyclic carbene (NHC) ligands acting as directing group as well as functionalizing unit in arene C–H functionalization reactions with alkynes, Choudhury and group [106] in 2015 developed a protocol for the
- intermolecular C–H annulation of NHC-substituted pyridines with a variety of internal alkynes 187 under rhodium catalysis for the synthesis of annulated and highly decorated pyridines 188 (Scheme 36). The authors used the N-heterocyclic carbene ligand as directing group to prepare imidazo[1,2-a][1,6

Graphical Abstract

Figure 1: Representative examples of bioactive natural products and FDA-approved drugs containing a pyridine ...

Scheme 1: Classical and traditional methods for the synthesis of functionalized pyridines.

Scheme 2: Rare earth metal (Ln)-catalyzed pyridine C–H alkylation.

Scheme 3: Pd-catalyzed C–H alkylation of pyridine N-oxide.

Scheme 4: CuI-catalyzed C–H alkylation of N-iminopyridinium ylides with tosylhydrazones (A) and a plausible r...

Scheme 5: Zirconium complex-catalyzed pyridine C–H alkylation.

Scheme 6: Rare earth metal-catalyzed pyridine C–H alkylation with nonpolar unsaturated substrates.

Scheme 7: Heterobimetallic Rh–Al complex-catalyzed ortho-C–H monoalkylation of pyridines.

Scheme 8: Mono(phosphinoamido)-rare earth complex-catalyzed pyridine C–H alkylation.

Scheme 9: Rhodium-catalyzed pyridine C–H alkylation with acrylates and acrylamides.

Scheme 10: Ni–Al bimetallic system-catalyzed pyridine C–H alkylation.

Scheme 11: Iridium-catalyzed pyridine C–H alkylation.

Scheme 12: para-C(sp2)–H Alkylation of pyridines with alkenes.

Scheme 13: Enantioselective pyridine C–H alkylation.

Scheme 14: Pd-catalyzed C2-olefination of pyridines.

Scheme 15: Ru-catalyzed C-6 (C-2)-propenylation of 2-arylated pyridines.

Scheme 16: C–H addition of allenes to pyridines catalyzed by half-sandwich Sc metal complex.

Scheme 17: Pd-catalyzed stereodivergent synthesis of alkenylated pyridines.

Scheme 18: Pd-catalyzed ligand-promoted selective C3-olefination of pyridines.

Scheme 19: Mono-N-protected amino acids in Pd-catalyzed C3-alkenylation of pyridines.

Scheme 20: Amide-directed and rhodium-catalyzed C3-alkenylation of pyridines.

Scheme 21: Bimetallic Ni–Al-catalyzed para-selective alkenylation of pyridine.

Scheme 22: Arylboronic ester-assisted pyridine direct C–H arylation.

Scheme 23: Pd-catalyzed C–H arylation/benzylation with toluene.

Scheme 24: Pd-catalyzed pyridine C–H arylation with potassium aryl- and heteroaryltrifluoroborates.

Scheme 25: Transient activator strategy in pyridine C–H biarylation.

Scheme 26: Ligand-promoted C3-arylation of pyridine.

Scheme 27: Pd-catalyzed arylation of nicotinic and isonicotinic acids.

Scheme 28: Iron-catalyzed and imine-directed C–H arylation of pyridines.

Scheme 29: Pd–(bipy-6-OH) cooperative system-mediated direct pyridine C3-arylation.

Scheme 30: Pd-catalyzed pyridine N-oxide C–H arylation with heteroarylcarboxylic acids.

Scheme 31: Pd-catalyzed C–H cross-coupling of pyridine N-oxides with five-membered heterocycles.

Scheme 32: Cu-catalyzed dehydrative biaryl coupling of azine(pyridine) N-oxides and oxazoles.

Scheme 33: Rh(III)-catalyzed cross dehydrogenative C3-heteroarylation of pyridines.

Scheme 34: Pd-catalyzed C3-selective arylation of pyridines.

Scheme 35: Rhodium-catalyzed oxidative C–H annulation of pyridines to quinolines.

Scheme 36: Rhodium-catalyzed and NHC-directed C–H annulation of pyridine.

Scheme 37: Ni/NHC-catalyzed regio- and enantioselective C–H cyclization of pyridines.

Scheme 38: Rare earth metal-catalyzed intramolecular C–H cyclization of pyridine to azaindolines.

Scheme 39: Rh-catalyzed alkenylation of bipyridine with terminal silylacetylenes.

Scheme 40: Rollover cyclometallation in Rh-catalyzed pyridine C–H functionalization.

Scheme 41: Rollover pathway in Rh-catalyzed C–H functionalization of N,N,N-tridentate chelating compounds.

Scheme 42: Pd-catalyzed rollover pathway in bipyridine-6-carboxamides C–H arylation.

Scheme 43: Rh-catalyzed C3-acylmethylation of bipyridine-6-carboxamides with sulfoxonium ylides.

Scheme 44: Rh-catalyzed C–H functionalization of bipyridines with alkynes.

Scheme 45: Rh-catalyzed C–H acylmethylation and annulation of bipyridine with sulfoxonium ylides.

Scheme 46: Iridium-catalyzed C4-borylation of pyridines.

Scheme 47: C3-Borylation of pyridines.

Scheme 48: Pd-catalyzed regioselective synthesis of silylated dihydropyridines.
Enolates ambushed – asymmetric tandem conjugate addition and subsequent enolate trapping with conventional and less traditional electrophiles
- Péter Kisszékelyi and
- Radovan Šebesta
Beilstein J. Org. Chem. 2023, 19, 593–634, doi:10.3762/bjoc.19.44
- , which begins with a Cu-catalyzed enantioselective conjugate addition/enolate alkylation tandem reaction sequence utilizing the N-heterocyclic carbene ligand L40 [112]. Ketone 232 was isolated in 61% yield and 81% ee. Hereafter, the authors were able to synthesize the complex tetracyclic structure within

Graphical Abstract

Scheme 1: General scheme depicting tandem reactions based on an asymmetric conjugate addition followed by an ...

Scheme 2: Cu-catalyzed tandem conjugate addition of R2Zn/aldol reaction with chiral acetals.

Scheme 3: Cu-catalyzed asymmetric desymmetrization of cyclopentene-1,3-diones using a tandem conjugate additi...

Scheme 4: Stereocontrolled assembly of dialkylzincs, cyclic enones, and sulfinylimines utilizing a Cu-catalyz...

Scheme 5: Cu-catalyzed tandem conjugate addition/Mannich reaction (A). Access to chiral isoindolinones and tr...

Scheme 6: Cu-catalyzed tandem conjugate addition/nitro-Mannich reaction (A) with syn–anti or syn–syn selectiv...

Figure 1: Various chiral ligands utilized for the tandem conjugate addition/Michael reaction sequences.

Scheme 7: Cu-catalyzed tandem conjugate addition/Michael reaction: side-product formation with chalcone (A) a...

Scheme 8: Zn enolate trapping using allyl iodides (A), Stork–Jung vinylsilane reagents (B), and allyl bromide...

Scheme 9: Cu-catalyzed tandem conjugate addition/acylation through Li R2Zn enolate (A). A four-component coup...

Scheme 10: Selected examples for the Cu-catalyzed tandem conjugate addition/trifluoromethylthiolation sequence....

Scheme 11: Zn enolates trapped by vinyloxiranes: synthesis of allylic alcohols.

Scheme 12: Stereoselective cyclopropanation of Mg enolates formed by ACA of Grignard reagents to chlorocrotona...

Scheme 13: Domino aldol reactions of Mg enolates formed from coumarin and chromone.

Scheme 14: Oxidative coupling of ACA-produced Mg enolates.

Scheme 15: Tandem ACA of Grignard reagents to enones and Mannich reaction.

Scheme 16: Diastereodivergent Mannich reaction of Mg enolates with differently N-protected imines.

Scheme 17: Tandem Grignard–ACA–Mannich using Taddol-based phosphine-phosphite ligands.

Scheme 18: Tandem reaction of Mg enolates with aminomethylating reagents.

Scheme 19: Tandem reaction composed of Grignard ACA to alkynyl enones.

Scheme 20: Rh/Cu-catalyzed tandem reaction of diazo enoates leading to cyclobutanes.

Scheme 21: Tandem Grignard-ACA of cyclopentenones and alkylation of enolates.

Scheme 22: Tandem ACA of Grignard reagents followed by enolate trapping reaction with onium compounds.

Scheme 23: Mg enolates generated from unsaturated lactones in reaction with activated alkenes.

Scheme 24: Lewis acid mediated ACA to amides and SN2 cyclization of a Br-appended enolate.

Scheme 25: Trapping reactions of aza-enolates with Michael acceptors.

Scheme 26: Si enolates generated by TMSOTf-mediated ACA of Grignard reagents and enolate trapping reaction wit...

Scheme 27: Trapping reactions of enolates generated from alkenyl heterocycles (A) and carboxylic acids (B) wit...

Scheme 28: Reactions of heterocyclic Mg enolates with onium compounds.

Scheme 29: Synthetic transformations of cycloheptatrienyl and benzodithiolyl substituents.

Scheme 30: Aminomethylation of Al enolates generated by ACA of trialkylaluminum reagents.

Scheme 31: Trapping reactions of enolates with activated alkenes.

Scheme 32: Alkynylation of racemic aluminum or magnesium enolates.

Scheme 33: Trapping reactions of Zr enolates generated by Cu-ACA of organozirconium reagents.

Scheme 34: Chloromethylation of Zr enolates using the Vilsmeier–Haack reagent.

Scheme 35: Tandem conjugate borylation with subsequent protonation or enolate trapping by an electrophile.

Scheme 36: Tandem conjugate borylation/aldol reaction of cyclohexenones.

Scheme 37: Selected examples for the tandem asymmetric borylation/intramolecular aldol reaction; synthesis of ...

Scheme 38: Cu-catalyzed tandem methylborylation of α,β-unsaturated phosphine oxide in the presence of (R,Sp)-J...

Scheme 39: Cu-catalyzed tandem transannular conjugated borylation/aldol cyclization of macrocycles containing ...

Scheme 40: Stereoselective tandem conjugate borylation/Mannich cyclization: selected examples (A) and a multi-...

Scheme 41: Some examples of Cu-catalyzed asymmetric tandem borylation/aldol cyclization (A). Application to di...

Scheme 42: Atropisomeric P,N-ligands used in tandem conjugate borylation/aldol cyclization sequence.

Scheme 43: Selected examples for the enantioselective Cu-catalyzed borylation/intramolecular Michael addition ...

Scheme 44: Selected examples for the preparation of enantioenriched spiroindanes using a Cu-catalyzed tandem c...

Scheme 45: Enantioselective conjugate borylation of cyclobutene-1-carboxylic acid diphenylmethyl ester 175 wit...

Scheme 46: Cu-catalyzed enantioselective tandem conjugate silylation of α,β-unsaturated ketones with subsequen...

Scheme 47: Cu-catalyzed enantioselective tandem conjugate silylation of α,β-unsaturated ketones with subsequen...

Scheme 48: Cu-catalyzed tandem conjugate silylation/aldol condensation. The diastereoselectivity is controlled...

Scheme 49: Chiral Ru-catalyzed three-component coupling reaction.

Scheme 50: Rh-Phebox complex-catalyzed reductive cyclization and subsequent reaction with Michael acceptors th...

Scheme 51: Rh-catalyzed tandem asymmetric conjugate alkynylation/aldol reaction (A) and subsequent spiro-cycli...

Scheme 52: Rh-bod complex-catalyzed tandem asymmetric conjugate arylation/intramolecular aldol addition (A). S...

Scheme 53: Co-catalyzed C–H-bond activation/asymmetric conjugate addition/aldol reaction.

Scheme 54: (Diisopinocampheyl)borane-promoted 1,4-hydroboration of α,β-unsaturated morpholine carboxamides and...

Figure 2: Some examples of total syntheses that have been recently reviewed.

Scheme 55: Stereoselective synthesis of antimalarial prodrug (+)-artemisinin utilizing a tandem conjugate addi...

Scheme 56: Amphilectane and serrulatane diterpenoids: preparation of chiral starting material via asymmetric t...

Scheme 57: Various asymmetric syntheses of pleuromutilin and related compounds based on a tandem conjugate add...

Scheme 58: Total synthesis of glaucocalyxin A utilizing a tandem conjugate addition/acylation reaction sequenc...

Scheme 59: Installation of the exocyclic double bond using a tandem conjugate addition/aminomethylation sequen...

Scheme 60: Synthesis of the taxol core using a tandem conjugate addition/enolate trapping sequence with Vilsme...

Scheme 61: Synthesis of the tricyclic core of 12-epi-JBIR-23/24 utilizing a Rh-catalyzed asymmetric conjugate ...

Scheme 62: Total synthesis of (−)-peyssonoside A utilizing a Cu-catalyzed enantioselective tandem conjugate ad...
Mechanochemical solid state synthesis of copper(I)/NHC complexes with K3PO4
- Ina Remy-Speckmann,
- Birte M. Zimmermann,
- Mahadeb Gorai,
- Martin Lerch and
- Johannes F. Teichert
Beilstein J. Org. Chem. 2023, 19, 440–447, doi:10.3762/bjoc.19.34
- , Germany 10.3762/bjoc.19.34 Abstract A protocol for the mechanochemical synthesis of copper(I)/N-heterocyclic carbene complexes using cheap and readily available K3PO4 as base has been developed. This method employing a ball mill is amenable to typical simple copper(I)/NHC complexes but also to a
- sophisticated copper(I)/N-heterocyclic carbene complex bearing a guanidine moiety. In this way, the present approach circumvents commonly employed silver(I) complexes which are associated with significant and undesired waste formation and the excessive use of solvents. The resulting bifunctional catalyst has
- focus has seldomly been on the preparative methods to access the required catalysts themselves. As case in point, we decided to re-investigate the synthesis of copper(I)/N-heterocyclic carbene (NHC) complexes, which are broadly applicable catalysts for a wide variety of transformations [4][5][6]. While

Graphical Abstract

Scheme 1: General synthetic routes to copper(I)/NHC complexes (X = Cl, Br).

Scheme 2: Preparation of sophisticated Cu(I)/NHC complexes: Synthesis of bifunctional catalyst 5 via transmet...

Scheme 3: Application of bifunctional catalyst 5 in copper(I)-catalyzed hydrogenations: comparison of 5 prepa...
Group 13 exchange and transborylation in catalysis
- Dominic R. Willcox and
- Stephen P. Thomas
Beilstein J. Org. Chem. 2023, 19, 325–348, doi:10.3762/bjoc.19.28
- /B‒H exchange [114]. Hevia reported a combination of a tris(alkyl)gallium species and bulky N-heterocyclic carbene acted as an FLP for B‒H insertion, and was used subsequently as a catalyst in the hydroboration of ketones, aldehydes, esters, and imines with HBpin [115]. Using an ONO-pincer-supported

Graphical Abstract

Scheme 1: Group 13 exchange.

Scheme 2: Borane-catalysed hydroboration of alkynes and the proposed mechanism.

Scheme 3: a) Borane-catalysed hydroboration of alkenes and the proposed mechanism. b) H-B-9-BBN-catalysed dou...

Scheme 4: a) Amine-borane-catalysed C‒H borylation of heterocycles and the proposed mechanism. b) Benzoic aci...

Scheme 5: Bis(pentafluorophenyl)borane-catalysed dimerisation of allenes and the proposed mechanism.

Scheme 6: Alkoxide-promoted hydroboration of heterocycles and the proposed mechanism.

Scheme 7: Borane-catalysed reduction of indoles and the proposed mechanism.

Scheme 8: H-B-9-BBN-catalysed hydrocyanation of enones and the proposed mechanism.

Scheme 9: Borane-catalysed hydroboration of nitriles and the proposed mechanism.

Scheme 10: Myrtanylborane-catalysed asymmetric reduction of propargylic ketones and the proposed mechanism.

Scheme 11: H-B-9-BBN-catalysed C–F esterification of alkyl fluorides and the proposed mechanism.

Scheme 12: H-B-9-BBN-catalysed 1,4-hydroboration of enones and the proposed mechanism.

Scheme 13: Boric acid-promoted reduction of esters, lactones, and carbonates and the proposed mechanism.

Scheme 14: H-B-9-BBN-catalysed reductive aldol-type reaction and the proposed mechanism.

Scheme 15: H-B-9-BBN-catalysed diastereoselective allylation of ketones and the Ph-BBD-catalysed enantioselect...

Scheme 16: H-B-9-BBN-catalysed C–F arylation of benzyl fluorides and the proposed mechanism.

Scheme 17: Borane-catalysed S‒H borylation of thiols and the proposed mechanism.

Scheme 18: Borane-catalysed hydroalumination of alkenes and allenes.

Scheme 19: a) Aluminium-catalysed hydroboration of alkynes and example catalysts. b) Deprotonation mechanistic...

Scheme 20: Aluminium-catalysed hydroboration of alkenes and the proposed mechanism.

Scheme 21: Aluminium-catalysed C–H borylation of terminal alkynes and the proposed mechanism.

Scheme 22: Aluminium-catalysed dehydrocoupling of amines, alcohols, and thiols with H-B-9-BBN or HBpin and the...

Scheme 23: Aluminium-catalysed hydroboration of unsaturated compounds and the general reaction mechanism.

Scheme 24: a) Gallium-catalysed asymmetric hydroboration of ketones and the proposed mechanism. b) Gallium-cat...

Scheme 25: Gallium(I)-catalysed allylation/propargylation of acetals and aminals and the proposed mechanism.

Scheme 26: Indium(I)-catalysed allylation/propargylation of acetals, aminals, and alkyl ethers.

Scheme 27: Iron–indium cocatalysed double hydroboration of nitriles and the proposed mechanism.

Figure 1: a) The number of reports for a given group 13 exchange in catalysis. b) Average free energy barrier...
Redox-active molecules as organocatalysts for selective oxidative transformations – an unperceived organocatalysis field
- Elena R. Lopat’eva,
- Igor B. Krylov,
- Dmitry A. Lapshin and
- Alexander O. Terent’ev
Beilstein J. Org. Chem. 2022, 18, 1672–1695, doi:10.3762/bjoc.18.179
- addition to the electron-rich C=C bond [58] or proton loss followed by β-functionalization [59][60][61]. The iminium cation catalysis is used in the activation of electrophilic properties of enones for the nucleophilic epoxidation by hydroperoxides (Scheme 2B). N-Heterocyclic carbene (NHC) organocatalysis
- -organocatalysts. Organocatalysis classification used in the present perspective. Oxidative processes catalyzed by amines. N-Heterocyclic carbene (NHC) catalysis in oxidative functionalization of aldehydes. Examples of asymmetric oxidative processes catalyzed by chiral Brønsted acids. Asymmetric aerobic α

Graphical Abstract

Scheme 1: Organocatalysis classification used in the present perspective.

Scheme 2: Oxidative processes catalyzed by amines.

Scheme 3: N-Heterocyclic carbene (NHC) catalysis in oxidative functionalization of aldehydes.

Scheme 4: Examples of asymmetric oxidative processes catalyzed by chiral Brønsted acids.

Scheme 5: Asymmetric aerobic α-hydroxylation of lactams under phase-transfer organocatalysis conditions emplo...

Scheme 6: Selective CH-oxidation of methylarenes to aldehydes or carboxylic acids.

Scheme 7: An example of the regioselective CH-amination by a sterically hindered imide-N-oxyl radical precurs...

Scheme 8: CH-amination of ethylbenzene and CH-fluorination of aldehydes catalyzed by N-hydroxybenzimidazoles,...

Scheme 9: Mixed hetero-/homogeneous TiO2/N-hydroxyimide photocatalysis in the selective benzylic oxidation.

Scheme 10: Electrochemical benzylic iodination and benzylation of pyridine by benzyl iodides generated in situ...

Scheme 11: Electrochemical oxidative C–O/C–N coupling of alkylarenes with NHPI. Electrolysis conditions: Const...

Scheme 12: Chemoselective alcohol oxidation catalyzed by TEMPO.

Scheme 13: ABNO-catalyzed oxidative C–N coupling of primary alcohols with primary amines.

Scheme 14: ACT-catalyzed electrochemical oxidation of primary alcohols and aldehydes to carboxylic acids.

Scheme 15: Electrocatalytic oxidation of benzylic alcohols by a TEMPO derivative immobilized on a graphite ano...

Scheme 16: Electrochemical oxidation of carbamates of cyclic amines to lactams and oxidative cyanation of amin...

Scheme 17: Hydrogen atom transfer (HAT) and single-electron transfer (SET) as basic principles of amine cation...

Scheme 18: Electrochemical quinuclidine-catalyzed oxidation involving unactivated C–H bonds.

Scheme 19: DABCO-mediated photocatalytic C–C cross-coupling involving aldehyde C–H bond cleavage.

Scheme 20: DABCO-derived cationic catalysts in inactivated C–H bond cleavage for alkyl radical addition to ele...

Scheme 21: Electrochemical diamination and dioxygenation of vinylarenes catalyzed by triarylamines.

Scheme 22: Electrochemical benzylic oxidation mediated by triarylimidazoles.

Scheme 23: Thiyl radical-catalyzed CH-arylation of allylic substrates by aryl cyanides.

Scheme 24: Synthesis of redox-active alkyl tetrafluoropyridinyl sulfides by unactivated C–H bond cleavage by t...

Scheme 25: Main intermediates in quinone oxidative organocatalysis.

Scheme 26: Electrochemical DDQ-catalyzed intramolecular dehydrogenative aryl–aryl coupling.

Scheme 27: DDQ-mediated cross-dehydrogenative C–N coupling of benzylic substrates with azoles.

Scheme 28: Biomimetic o-quinone-catalyzed benzylic alcohol oxidation.

Scheme 29: Electrochemical synthesis of secondary amines by oxidative coupling of primary amines and benzylic ...

Scheme 30: General scheme of dioxirane and oxaziridine oxidative organocatalysis.

Scheme 31: Dioxirane organocatalyzed CH-hydroxylation involving aliphatic C(sp3)–H bonds.

Scheme 32: Enantioselective hydroxylation of CH-acids catalyzed by chiral oxaziridines.

Scheme 33: Iodoarene-organocatalyzed vinylarene diamination.

Scheme 34: Iodoarene-organocatalyzed asymmetric CH-hydroxylation of benzylic substrates.

Scheme 35: Iodoarene-organocatalyzed asymmetric difluorination of alkenes with migration of aryl or methyl gro...

Scheme 36: Examples of 1,2-diiodo-4,5-dimethoxybenzene-catalyzed electrochemical oxidative heterocyclizations.

Scheme 37: Electrochemical N-ammonium ylide-catalyzed CH-oxidation.

Scheme 38: Oxidative dimerization of aryl- and alkenylmagnesium compounds catalyzed by quinonediimines.

Scheme 39: FLP-catalyzed dehydrogenation of N-substituted indolines.
First example of organocatalysis by cathodic N-heterocyclic carbene generation and accumulation using a divided electrochemical flow cell
- Daniele Rocco,
- Ana A. Folgueiras-Amador,
- Richard C. D. Brown and
- Marta Feroci
Beilstein J. Org. Chem. 2022, 18, 979–990, doi:10.3762/bjoc.18.98
- used as organocatalyst in two classical umpolung reactions of cinnamaldehyde: its cyclodimerization and its oxidative esterification. Keywords: Breslow intermediate; cathodic reduction; flow electrochemistry; N-heterocyclic carbene; oxidative esterification; Introduction Ionic liquids (ILs) are well
- can be modified by the presence of a base or by a single electron cathodic reduction of the C–H between nitrogen atoms of the imidazolium ring (Scheme 1), inducing the formation of a N-heterocyclic carbene (NHC) [7][8]. In recent years, NHCs have achieved great success: they have been frequently used
- reduction of an imidazolium cation to NHC, in an undivided cell under flow conditions, coupled with the anodic generation of Cu(I) from a sacrificial anode to yield the corresponding N-heterocyclic carbene complex [34][35]. In this case, irreversible capture of the NHC by the metallic cation prevented NHC

Graphical Abstract

Scheme 1: Electrochemical generation of NHC.

Scheme 2: Transformation of electrochemically generated NHC into the corresponding thione by its reaction wit...

Scheme 3: Umpolung of the aldehyde carbonyl carbon atom. Formation of the Breslow intermediate using NHCs.

Figure 1: Schematic representation of a plane-parallel plate flow electrochemical reactor.

Figure 2: C/PVDF anode before (A) and after (B) the first experiment (Table 1, entry 1).

Scheme 4: Electrogenerated NHC-catalyzed self-annulation of cinnamaldehyde.

Scheme 5: Byproduct obtained from the reaction between methanol and the Breslow intermediate.

Figure 3: Expanded view of the electrochemical cell components: (a) Aluminium end plates; (b) insulating PTFE...
A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries
- Guido Gambacorta,
- James S. Sharley and
- Ian R. Baxendale
Beilstein J. Org. Chem. 2021, 17, 1181–1312, doi:10.3762/bjoc.17.90

Graphical Abstract

Figure 1: Representative shares of the global F&F market (2018) segmented on their applications [1].

Figure 2: General structure of an international fragrance company [2].

Figure 3: The Michael Edwards fragrance wheel.

Figure 4: Examples of oriental (1–3), woody (4–7), fresh (8–10), and floral (11 and 12) notes.

Figure 5: A basic depiction of batch vs flow.

Scheme 1: Examples of reactions for which flow processing outperforms batch.

Scheme 2: Some industrially important aldol-based transformations.

Scheme 3: Biphasic continuous aldol reactions of acetone and various aldehydes.

Scheme 4: Aldol synthesis of 43 in flow using LiHMDS as the base.

Scheme 5: A semi-continuous synthesis of doravirine (49) involving a key aldol reaction.

Scheme 6: Enantioselective aldol reaction using 5-(pyrrolidin-2-yl)tetrazole (51) as catalyst in a microreact...

Scheme 7: Gröger's example of asymmetric aldol reaction in aqueous media.

Figure 6: Immobilised reagent column reactor types.

Scheme 8: Photoinduced thiol–ene coupling preparation of silica-supported 5-(pyrrolidin-2-yl)tetrazole 63 and...

Scheme 9: Continuous-flow approach for enantioselective aldol reactions using the supported catalyst 67.

Scheme 10: Ötvös’ employment of a solid-supported peptide aldol catalyst in flow.

Scheme 11: The use of proline tetrazole packed in a column for aldol reaction between cyclohexanone (65) and 2...

Scheme 12: Schematic diagram of an aminosilane-grafted Si-Zr-Ti/PAI-HF reactor for continuous-flow aldol and n...

Scheme 13: Continuous-flow condensation for the synthesis of the intermediate 76 to nabumetone (77) and Microi...

Scheme 14: Synthesis of ψ-Ionone (80) in continuous-flow via aldol condensation between citral (79) and aceton...

Scheme 15: Synthesis of β-methyl-ionones (83) from citral (79) in flow. The steps are separately described, an...

Scheme 16: Continuous-flow synthesis of 85 from 84 described by Gavriilidis et al.

Scheme 17: Continuous-flow scCO2 apparatus for the synthesis of 2-methylpentanal (87) and the self-condensed u...

Scheme 18: Chen’s two-step flow synthesis of coumarin (90).

Scheme 19: Pechmann condensation for the synthesis of 7-hydroxyxcoumarin (93) in flow. The setup extended to c...

Scheme 20: Synthesis of the dihydrojasmonate 35 exploiting nitro derivative proposed by Ballini et al.

Scheme 21: Silica-supported amines as heterogeneous catalyst for nitroaldol condensation in flow.

Scheme 22: Flow apparatus for the nitroaldol condensation of p-hydroxybenzaldehyde (102) to nitrostyrene 103 a...

Scheme 23: Nitroaldol reaction of 64 to 105 employing a quaternary ammonium functionalised PANF.

Scheme 24: Enantioselective nitroaldol condensation for the synthesis of 108 under flow conditions.

Scheme 25: Enatioselective synthesis of 1,2-aminoalcohol 110 via a copper-catalysed nitroaldol condensation.

Scheme 26: Examples of Knoevenagel condensations applied for fragrance components.

Scheme 27: Flow apparatus for Knoevenagel condensation described in 1989 by Venturello et al.

Scheme 28: Knoevenagel reaction using a coated multichannel membrane microreactor.

Scheme 29: Continuous-flow apparatus for Knoevenagel condensation employing sugar cane bagasse as support deve...

Scheme 30: Knoevenagel reaction for the synthesis of 131–135 in flow using an amine-functionalised silica gel. ...

Scheme 31: Continuous-flow synthesis of compound 137, a key intermediate for the synthesis of pregabalin (138)...

Scheme 32: Continuous solvent-free apparatus applied for the synthesis of compounds 140–143 using a TSE. Throu...

Scheme 33: Lewis et al. developed a spinning disc reactor for Darzens condensation of 144 and a ketone to furn...

Scheme 34: Some key industrial applications of conjugate additions in the F&F industry.

Scheme 35: Continuous-flow synthesis of 4-(2-hydroxyethyl)thiomorpholine 1,1-dioxide (156) via double conjugat...

Scheme 36: Continuous-flow system for Michael addition using CsF on alumina as the catalyst.

Scheme 37: Calcium chloride-catalysed asymmetric Michael addition using an immobilised chiral ligand.

Scheme 38: Continuous multistep synthesis for the preparation of (R)-rolipram (173). Si-NH2: primary amine-fun...

Scheme 39: Continuous-flow Michael addition using ion exchange resin Amberlyst® A26.

Scheme 40: Preparation of the heterogeneous catalyst 181 developed by Paixão et al. exploiting Ugi multicompon...

Scheme 41: Continuous-flow system developed by the Paixão’s group for the preparation of Michael asymmetric ad...

Scheme 42: Continuous-flow synthesis of nitroaldols catalysed by supported catalyst 184 developed by Wennemers...

Scheme 43: Heterogenous polystyrene-supported catalysts developed by Pericàs and co-workers.

Scheme 44: PANF-supported pyrrolidine catalyst for the conjugate addition of cyclohexanone (65) and trans-β-ni...

Scheme 45: Synthesis of (−)-paroxetine precursor 195 developed by Ötvös, Pericàs, and Kappe.

Scheme 46: Continuous-flow approach for the 5-step synthesis of (−)-oseltamivir (201) as devised by Hayashi an...

Scheme 47: Continuous-flow enzyme-catalysed Michael addition.

Scheme 48: Continuous-flow copper-catalysed 1,4 conjugate addition of Grignard reagents to enones. Reprinted w...

Scheme 49: A collection of commonly encountered hydrogenation reactions.

Figure 7: The ThalesNano H-Cube® continuous-flow hydrogenator.

Scheme 50: Chemoselective reduction of an α,β-unsaturated ketone using the H-Cube® reactor.

Scheme 51: Incorporation of Lindlar’s catalyst into the H-Cube® reactor for the reduction of an alkyne.

Scheme 52: Continuous-flow semi-hydrogenation of alkyne 208 to 209 using SACs with H-Cube® system.

Figure 8: The standard setups for tube-in-tube gas–liquid reactor units.

Scheme 53: Homogeneous hydrogenation of olefins using a tube-in-tube reactor setup.

Scheme 54: Recyclable heterogeneous flow hydrogenation system.

Scheme 55: Leadbeater’s reverse tube-in-tube hydrogenation system for olefin reductions.

Scheme 56: a) Hydrogenation using a Pd-immobilised microchannel reactor (MCR) and b) a representation of the i...

Scheme 57: Hydrogenation of alkyne 238 exploiting segmented flow in a Pd-immobilised capillary reactor.

Scheme 58: Continuous hydrogenation system for the preparation of cyrene (241) from (−)-levoglucosenone (240).

Scheme 59: Continuous hydrogenation system based on CSMs developed by Hornung et al.

Scheme 60: Chemoselective reduction of carbonyls (ketones over aldehydes) in flow.

Scheme 61: Continuous system for the semi-hydrogenation of 256 and 258, developed by Galarneau et al.

Scheme 62: Continuous synthesis of biodiesel fuel 261 from lignin-derived furfural acetone (260).

Scheme 63: Continuous synthesis of γ-valerolacetone (263) via CTH developed by Pineda et al.

Scheme 64: Continuous hydrogenation of lignin-derived biomass (products 265, 266, and 267) using a sustainable...

Scheme 65: Ru/C or Rh/C-catalysed hydrogenation of arene in flow as developed by Sajiki et al.

Scheme 66: Polysilane-immobilized Rh–Pt-catalysed hydrogenation of arenes in flow by Kobayashi et al.

Scheme 67: High-pressure in-line mixing of H2 for the asymmetric reduction of 278 at pilot scale with a 73 L p...

Figure 9: Picture of the PFR employed at Eli Lilly & Co. for the continuous hydrogenation of 278 [287]. Reprinted ...

Scheme 68: Continuous-flow asymmetric hydrogenation using Oppolzer's sultam 280 as chiral auxiliary.

Scheme 69: Some examples of industrially important oxidation reactions in the F&F industry. CFL: compact fluor...

Scheme 70: Gold-catalysed heterogeneous oxidation of alcohols in flow.

Scheme 71: Uozumi’s ARP-Pt flow oxidation protocol.

Scheme 72: High-throughput screening of aldehyde oxidation in flow using an in-line GC.

Scheme 73: Permanganate-mediated Nef oxidation of nitroalkanes in flow with the use of in-line sonication to p...

Scheme 74: Continuous-flow aerobic anti-Markovnikov Wacker oxidation.

Scheme 75: Continuous-flow oxidation of 2-benzylpyridine (312) using air as the oxidant.

Scheme 76: Continuous-flow photo-oxygenation of monoterpenes.

Scheme 77: A tubular reactor design for flow photo-oxygenation.

Scheme 78: Glucose oxidase (GOx)-mediated continuous oxidation of glucose using compressed air and the FFMR re...

Scheme 79: Schematic continuous-flow sodium hypochlorite/TEMPO oxidation of alcohols.

Scheme 80: Oxidation using immobilised TEMPO (344) was developed by McQuade et al.

Scheme 81: General protocol for the bleach/catalytic TBAB oxidation of aldehydes and alcohols.

Scheme 82: Continuous-flow PTC-assisted oxidation using hydrogen peroxide. The process was easily scaled up by...

Scheme 83: Continuous-flow epoxidation of cyclohexene (348) and in situ preparation of m-CPBA.

Scheme 84: Continuous-flow epoxidation using DMDO as oxidant.

Scheme 85: Mukayama aerobic epoxidation optimised in flow mode by the Favre-Réguillon group.

Scheme 86: Continuous-flow asymmetric epoxidation of derivatives of 359 exploiting a biomimetic iron catalyst.

Scheme 87: Continuous-flow enzymatic epoxidation of alkenes developed by Watts et al.

Scheme 88: Engineered multichannel microreactor for continuous-flow ozonolysis of 366.

Scheme 89: Continuous-flow synthesis of the vitamin D precursor 368 using multichannel microreactors. MFC: mas...

Scheme 90: Continuous ozonolysis setup used by Kappe et al. for the synthesis of various substrates employing ...

Scheme 91: Continuous-flow apparatus for ozonolysis as developed by Ley et al.

Scheme 92: Continuous-flow ozonolysis for synthesis of vanillin (2) using a film-shear flow reactor.

Scheme 93: Examples of preparative methods for ajoene (386) and allicin (388).

Scheme 94: Continuous-flow oxidation of thioanisole (389) using styrene-based polymer-supported peroxytungstat...

Scheme 95: Continuous oxidation of thiosulfinates using Oxone®-packed reactor.

Scheme 96: Continuous-flow electrochemical oxidation of thioethers.

Scheme 97: Continuous-flow oxidation of 400 to cinnamophenone (235).

Scheme 98: Continuous-flow synthesis of dehydrated material 401 via oxidation of methyl dihydrojasmonate (33).

Scheme 99: Some industrially important transformations involving Grignard reagents.

Scheme 100: Grachev et al. apparatus for continuous preparation of Grignard reagents.

Scheme 101: Example of fluidized Mg bed reactor with NMR spectrometer as on-line monitoring system.

Scheme 102: Continuous-flow synthesis of Grignard reagents and subsequent quenching reaction.

Figure 10: Membrane-based, liquid–liquid separator with integrated pressure control [52]. Adapted with permission ...

Scheme 103: Continuous-flow synthesis of 458, an intermediate to fluconazole (459).

Scheme 104: Continuous-flow synthesis of ketones starting from benzoyl chlorides.

Scheme 105: A Grignard alkylation combining CSTR and PFR technologies with in-line infrared reaction monitoring....

Scheme 106: Continuous-flow preparation of 469 from Grignard addition of methylmagnesium bromide.

Scheme 107: Continuous-flow synthesis of Grignard reagents 471.

Scheme 108: Preparation of the Grignard reagent 471 using CSTR and the continuous process for synthesis of the ...

Scheme 109: Continuous process for carboxylation of Grignard reagents in flow using tube-in-tube technology.

Scheme 110: Continuous synthesis of propargylic alcohols via ethynyl-Grignard reagent.

Scheme 111: Silica-supported catalysed enantioselective arylation of aldehydes using Grignard reagents in flow ...

Scheme 112: Acid-catalysed rearrangement of citral and dehydrolinalool derivatives.

Scheme 113: Continuous stilbene isomerisation with continuous recycling of photoredox catalyst.

Scheme 114: Continuous-flow synthesis of compound 494 as developed by Ley et al.

Scheme 115: Selected industrial applications of DA reaction.

Scheme 116: Multistep flow synthesis of the spirocyclic structure 505 via employing DA cycloaddition.

Scheme 117: Continuous-flow DA reaction developed in a plater flow reactor for the preparation of the adduct 508...

Scheme 118: Continuous-flow DA reaction using a silica-supported imidazolidinone organocatalyst.

Scheme 119: Batch vs flow for the DA reaction of (cyclohexa-1,5-dien-1-yloxy)trimethylsilane (513) with acrylon...

Scheme 120: Continuous-flow DA reaction between 510 and 515 using a shell-core droplet system.

Scheme 121: Continuous-flow synthesis of bicyclic systems from benzyne precursors.

Scheme 122: Continuous-flow synthesis of bicyclic scaffolds 527 and 528 for further development of potential ph...

Scheme 123: Continuous-flow inverse-electron hetero-DA reaction to pyridine derivatives such as 531.

Scheme 124: Comparison between batch and flow for the synthesis of pyrimidinones 532–536 via retro-DA reaction ...

Scheme 125: Continuous-flow coupled with ultrasonic system for preparation of ʟ-ascorbic acid derivatives 539 d...

Scheme 126: Two-step continuous-flow synthesis of triazole 543.

Scheme 127: Continuous-flow preparation of triazoles via CuAAC employing 546-based heterogeneous catalyst.

Scheme 128: Continuous-flow synthesis of compounds 558 through A3-coupling and 560 via AgAAC both employing the...

Scheme 129: Continuous-flow photoinduced [2 + 2] cycloaddition for the preparation of bicyclic derivatives of 5...

Scheme 130: Continuous-flow [2 + 2] and [5 + 2] cycloaddition on large scale employing a flow reactor developed...

Scheme 131: Continuous-flow preparation of the tricyclic structures 573 and 574 starting from pyrrole 570 via [...

Scheme 132: Continuous-flow [2 + 2] photocyclization of cinnamates.

Scheme 133: Continuous-flow preparation of cyclobutane 580 on a 5-plates photoreactor.

Scheme 134: Continuous-flow [2 + 2] photocycloaddition under white LED lamp using heterogeneous PCN as photocat...

Figure 11: Picture of the parallel tube flow reactor (PTFR) "The Firefly" developed by Booker-Milburn et al. a...

Scheme 135: Continuous-flow acid-catalysed [2 + 2] cycloaddition between silyl enol ethers and acrylic esters.

Scheme 136: Continuous synthesis of lactam 602 using glass column reactors.

Scheme 137: In situ generation of ketenes for the Staudinger lactam synthesis developed by Ley and Hafner.

Scheme 138: Application of [2 + 2 + 2] cycloadditions in flow employed by Ley et al.

Scheme 139: Examples of FC reactions applied in F&F industry.

Scheme 140: Continuous-flow synthesis of ibuprofen developed by McQuade et al.

Scheme 141: The FC acylation step of Jamison’s three-step ibuprofen synthesis.

Scheme 142: Synthesis of naphthalene derivative 629 via FC acylation in microreactors.

Scheme 143: Flow system for rapid screening of catalysts and reaction conditions developed by Weber et al.

Scheme 144: Continuous-flow system developed by Buorne, Muller et al. for DSD optimisation of the FC acylation ...

Scheme 145: Continuous-flow FC acylation of alkynes to yield β-chlorovinyl ketones such as 638.

Scheme 146: Continuous-flow synthesis of tonalide (619) developed by Wang et al.

Scheme 147: Continuous-flow preparation of acylated arene such as 290 employing Zr4+-β-zeolite developed by Kob...

Scheme 148: Flow system applied on an Aza-FC reaction catalysed by the thiourea catalyst 648.

Scheme 149: Continuous hydroformylation in scCO2.

Scheme 150: Two-step flow synthesis of aldehyde 655 through a sequential Heck reaction and subsequent hydroform...

Scheme 151: Single-droplet (above) and continuous (below) flow reactors developed by Abolhasani et al. for the ...

Scheme 152: Continuous hydroformylation of 1-dodecene (655) using a PFR-CSTR system developed by Sundmacher et ...

Scheme 153: Continuous-flow synthesis of the aldehyde 660 developed by Eli Lilly & Co. [32]. Adapted with permissio...

Scheme 154: Continuous asymmetric hydroformylation employing heterogenous catalst supported on carbon-based sup...

Scheme 155: Examples of acetylation in F&F industry: synthesis of bornyl (S,R,S-664) and isobornyl (S,S,S-664) ...

Scheme 156: Continuous-flow preparation of bornyl acetate (S,R,S-664) employing the oscillating flow reactor.

Scheme 157: Continuous-flow synthesis of geranyl acetate (666) from acetylation of geraniol (343) developed by ...

Scheme 158: 12-Ttungstosilicic acid-supported silica monolith-catalysed acetylation in flow.

Scheme 159: Continuous-flow preparation of cyclopentenone 676.

Scheme 160: Two-stage synthesis of coumarin (90) via acetylation of salicylaldehyde (88).

Scheme 161: Intensification process for acetylation of 5-methoxytryptamine (677) to melatonin (678) developed b...

Scheme 162: Examples of macrocyclic musky odorants both natural (679–681) and synthetic (682 and 683).

Scheme 163: Flow setup combined with microwave for the synthesis of macrocycle 686 via RCM.

Scheme 164: Continuous synthesis of 2,5-dihydro-1H-pyrroles via ring-closing metathesis.

Scheme 165: Continuous-flow metathesis of 485 developed by Leadbeater et al.

Figure 12: Comparison between RCM performed using different routes for the preparation of 696. On the left the...

Scheme 166: Continuous-flow RCM of 697 employed the solid-supported catalyst 698 developed by Grela, Kirschning...

Scheme 167: Continuous-flow RORCM of cyclooctene employing the silica-absorbed catalyst 700.

Scheme 168: Continuous-flow self-metathesis of methyl oleate (703) employing SILP catalyst 704.

Scheme 169: Flow apparatus for the RCM of 697 using a nanofiltration membrane for the recovery and reuse of the...

Scheme 170: Comparison of loadings between RCMs performed with different routes for the synthesis of 709.
Valorisation of plastic waste via metal-catalysed depolymerisation
- Francesca Liguori,
- Carmen Moreno-Marrodán and
- Pierluigi Barbaro
Beilstein J. Org. Chem. 2021, 17, 589–621, doi:10.3762/bjoc.17.53

- temperature (35 °C) but toxic CH2Cl2 solvent (Scheme 1). The reaction using second-generation N-heterocyclic carbene ligands was faster and preferably yielded cyclododecatriene. Larger cyclic butadienes may be used in the production of flame retardants, lubricants and specialty polymers [149][150]. 3.1.3
- electronic effect of the ligand [267]. A reaction mechanism for PLA depolymerisation was proposed, consisting of two consecutive first-order steps, in which Me-La production follows the formation of chain-end groups intermediates [265]. A zinc–N-heterocyclic carbene complex was used as catalysts for the

Graphical Abstract

Figure 1: Potential classification of plastic recycling processes. The area covered by the present review is ...

Figure 2: EG produced during glycolytic depolymerisation of PET using DEG + DPG as solvent and titanium(IV) n...

Scheme 1: Simplified representation of the conversion of 1,4-PBD to C16–C44 macrocycles using Ru metathesis c...

Figure 3: Main added-value monomers obtainable by catalytic depolymerisation of PET via chemolytic methods.

Scheme 2: Hydrogenolytic depolymerisation of PET by ruthenium complexes.

Scheme 3: Depolymerisation of PET via catalytic hydrosilylation by Ir(III) pincer complex.

Scheme 4: Catalytic hydrolysis (top) and methanolysis (bottom) reactions of PET.

Scheme 5: Depolymerisation of PET by glycolysis with ethylene glycol.

Figure 4: Glycolysis of PET: evolution of BHET yield over time, with and without zinc acetate catalyst (196 °...

Scheme 6: Potential activated complex for the glycolysis reaction of PET catalysed by metallated ILs and evol...

Scheme 7: One-pot, two-step process for PET repurposing via chemical recycling.

Scheme 8: Synthetic routes to PLA.

Scheme 9: Structures of the zinc molecular catalysts used for PLA-methanolysis in various works. a) See [265], b) ...

Scheme 10: Depolymerisation of PLLA by Zn–N-heterocyclic carbene complex.

Scheme 11: Salalen ligands.

Scheme 12: Catalytic hydrogenolysis of PLA.

Scheme 13: Catalytic hydrosilylation of PLA.

Scheme 14: Hydrogenative depolymerisation of PBT and PCL by molecular Ru catalysts.

Scheme 15: Glycolysis reaction of PCT by diethylene glycol.

Scheme 16: Polymerisation–depolymerisation cycle of 3,4-T6GBL.

Scheme 17: Polymerisation–depolymerisation cycle of 2,3-HDB.

Scheme 18: Hydrogenative depolymerisation of PBPAC by molecular Ru catalysts.

Scheme 19: Catalytic hydrolysis (top), alcoholysis (middle) and aminolysis (bottom) reactions of PBPAC.

Scheme 20: Hydrogenative depolymerisation of PPC (top) and PEC (bottom) by molecular Ru catalysts.

Scheme 21: Polymerisation-depolymerisation cycle of BEP.

Scheme 22: Hydrogenolysis of polyamides using soluble Ru catalysts.

Scheme 23: Catalytic depolymerisation of epoxy resin/carbon fibres composite.

Scheme 24: Depolymerisation of polyethers with metal salt catalysts and acyl chlorides.

Scheme 25: Proposed mechanism for the iron-catalysed depolymerisation reaction of polyethers. Adapted with per...
Recent developments in enantioselective photocatalysis
- Callum Prentice,
- James Morrisson,
- Andrew D. Smith and
- Eli Zysman-Colman
Beilstein J. Org. Chem. 2020, 16, 2363–2441, doi:10.3762/bjoc.16.197

- 100 in good yields and enantioselectivities (21 examples for THCs, up to 98:2 er and 10 examples for THIQs, up to 98:2 er). N-Heterocyclic carbene catalysis N-Heterocyclic carbene (NHC) catalysis was first used in combination with photoredox catalysis by Rovis in 2012. They showed that iminium ions

Graphical Abstract

Scheme 1: Amine/photoredox-catalysed α-alkylation of aldehydes with alkyl bromides bearing electron-withdrawi...

Scheme 2: Amine/HAT/photoredox-catalysed α-functionalisation of aldehydes using alkenes.

Scheme 3: Amine/cobalt/photoredox-catalysed α-functionalisation of ketones and THIQs.

Scheme 4: Amine/photoredox-catalysed α-functionalisation of aldehydes or ketones with imines. (a) Using keton...

Scheme 5: Bifunctional amine/photoredox-catalysed enantioselective α-functionalisation of aldehydes.

Scheme 6: Bifunctional amine/photoredox-catalysed α-functionalisation of aldehydes using amine catalysts via ...

Scheme 7: Amine/photoredox-catalysed RCA of iminium ion intermediates. (a) Synthesis of quaternary stereocent...

Scheme 8: Bifunctional amine/photoredox-catalysed RCA of enones in a radical chain reaction initiated by an i...

Scheme 9: Bifunctional amine/photoredox-catalysed RCA reactions of iminium ions with different radical precur...

Scheme 10: Bifunctional amine/photoredox-catalysed radical cascade reactions between enones and alkenes with a...

Scheme 11: Amine/photocatalysed photocycloadditions of iminium ion intermediates. (a) External photocatalyst u...

Scheme 12: Amine/photoredox-catalysed addition of acrolein (94) to iminium ions.

Scheme 13: Dual NHC/photoredox-catalysed acylation of THIQs.

Scheme 14: NHC/photocatalysed spirocyclisation via photoisomerisation of an extended Breslow intermediate.

Scheme 15: CPA/photoredox-catalysed aza-pinacol cyclisation.

Scheme 16: CPA/photoredox-catalysed Minisci-type reaction between azaarenes and α-amino radicals.

Scheme 17: CPA/photoredox-catalysed radical additions to azaarenes. (a) α-Amino radical or ketyl radical addit...

Scheme 18: CPA/photoredox-catalysed reduction of azaarene-derived substrates. (a) Reduction of ketones. (b) Ex...

Scheme 19: CPA/photoredox-catalysed radical coupling reactions of α-amino radicals with α-carbonyl radicals. (...

Scheme 20: CPA/photoredox-catalysed Povarov reaction.

Scheme 21: CPA/photoredox-catalysed reactions with imines. (a) Decarboxylative imine generation followed by Po...

Scheme 22: Bifunctional CPA/photocatalysed [2 + 2] photocycloadditions.

Scheme 23: PTC/photocatalysed oxygenation of 1-indanone-derived β-keto esters.

Scheme 24: PTC/photoredox-catalysed perfluoroalkylation of 1-indanone-derived β-keto esters via a radical chai...

Scheme 25: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloadditions of quinolon...

Scheme 26: Bifunctional hydrogen bonding/photocatalysed intramolecular RCA cyclisation of a quinolone.

Scheme 27: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloadditions of quinolon...

Scheme 28: Bifunctional hydrogen bonding/photocatalysed [2 + 2] photocycloaddition reactions. (a) First use of...

Scheme 29: Bifunctional hydrogen bonding/photocatalysed deracemisation of allenes.

Scheme 30: Bifunctional hydrogen bonding/photocatalysed deracemisation reactions. (a) Deracemisation of sulfox...

Scheme 31: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloaddition of coumarins....

Scheme 32: Bifunctional hydrogen bonding/photocatalysed [2 + 2] photocycloadditions of quinolones. (a) Intramo...

Scheme 33: Hydrogen bonding/photocatalysed formal arylation of benzofuranones.

Scheme 34: Hydrogen bonding/photoredox-catalysed dehalogenative protonation of α,α-chlorofluoro ketones.

Scheme 35: Hydrogen bonding/photoredox-catalysed reductions. (a) Reduction of 1,2-diketones. (b) Reduction of ...

Scheme 36: Hydrogen bonding/HAT/photocatalysed deracemisation of cyclic ureas.

Scheme 37: Hydrogen bonding/HAT/photoredox-catalysed synthesis of cyclic sulfonamides.

Scheme 38: Hydrogen bonding/photoredox-catalysed reaction between imines and indoles.

Scheme 39: Chiral cation/photoredox-catalysed radical coupling of two α-amino radicals.

Scheme 40: Chiral phosphate/photoredox-catalysed hydroetherfication of alkenols.

Scheme 41: Chiral phosphate/photoredox-catalysed synthesis of pyrroloindolines.

Scheme 42: Chiral anion/photoredox-catalysed radical cation Diels–Alder reaction.

Scheme 43: Lewis acid/photoredox-catalysed cycloadditions of carbonyls. (a) Formal [2 + 2] cycloaddition of en...

Scheme 44: Lewis acid/photoredox-catalysed RCA reaction using a scandium Lewis acid between α-amino radicals a...

Scheme 45: Lewis acid/photoredox-catalysed RCA reaction using a copper Lewis acid between α-amino radicals and...

Scheme 46: Lewis acid/photoredox-catalysed synthesis of 1,2-amino alcohols from aldehydes and nitrones using a...

Scheme 47: Lewis acid/photocatalysed [2 + 2] photocycloadditions of enones and alkenes.

Scheme 48: Meggers’s chiral-at-metal catalysts.

Scheme 49: Lewis acid/photoredox-catalysed α-functionalisation of ketones with alkyl bromides bearing electron...

Scheme 50: Bifunctional Lewis acid/photoredox-catalysed radical coupling reaction using α-chloroketones and α-...

Scheme 51: Lewis acid/photocatalysed RCA of enones. (a) Using aldehydes as acyl radical precursors. (b) Other ...

Scheme 52: Bifunctional Lewis acid/photocatalysis for a photocycloaddition of enones.

Scheme 53: Lewis acid/photoredox-catalysed RCA reactions of enones using DHPs as radical precursors.

Scheme 54: Lewis acid/photoredox-catalysed functionalisation of β-ketoesters. (a) Hydroxylation reaction catal...

Scheme 55: Bifunctional copper-photocatalysed alkylation of imines.

Scheme 56: Copper/photocatalysed alkylation of imines. (a) Bifunctional copper catalysis using α-silyl amines....

Scheme 57: Bifunctional Lewis acid/photocatalysed intramolecular [2 + 2] photocycloaddition.

Scheme 58: Bifunctional Lewis acid/photocatalysed [2 + 2] photocycloadditions (a) Intramolecular cycloaddition...

Scheme 59: Bifunctional Lewis acid/photocatalysed rearrangement of 2,4-dieneones.

Scheme 60: Lewis acid/photocatalysed [2 + 2] cycloadditions of cinnamate esters and styrenes.

Scheme 61: Nickel/photoredox-catalysed arylation of α-amino acids using aryl bromides.

Scheme 62: Nickel/photoredox catalysis. (a) Desymmetrisation of cyclic meso-anhydrides using benzyl trifluorob...

Scheme 63: Nickel/photoredox catalysis for the acyl-carbamoylation of alkenes with aldehydes using TBADT as a ...

Scheme 64: Bifunctional copper/photoredox-catalysed C–N coupling between α-chloro amides and carbazoles or ind...

Scheme 65: Bifunctional copper/photoredox-catalysed difunctionalisation of alkenes with alkynes and alkyl or a...

Scheme 66: Copper/photoredox-catalysed decarboxylative cyanation of benzyl phthalimide esters.

Scheme 67: Copper/photoredox-catalysed cyanation reactions using TMSCN. (a) Propargylic cyanation (b) Ring ope...

Scheme 68: Palladium/photoredox-catalysed allylic alkylation reactions. (a) Using alkyl DHPs as radical precur...

Scheme 69: Manganese/photoredox-catalysed epoxidation of terminal alkenes.

Scheme 70: Chromium/photoredox-catalysed allylation of aldehydes.

Scheme 71: Enzyme/photoredox-catalysed dehalogenation of halolactones.

Scheme 72: Enzyme/photoredox-catalysed dehalogenative cyclisation.

Scheme 73: Enzyme/photoredox-catalysed reduction of cyclic imines.

Scheme 74: Enzyme/photocatalysed enantioselective reduction of electron-deficient alkenes as mixtures of (E)/(Z...

Scheme 75: Enzyme/photoredox catalysis. (a) Deacetoxylation of cyclic ketones. (b) Reduction of heteroaromatic...

Scheme 76: Enzyme/photoredox-catalysed synthesis of indole-3-ones from 2-arylindoles.

Scheme 77: Enzyme/HAT/photoredox catalysis for the DKR of primary amines.

Scheme 78: Bifunctional enzyme/photoredox-catalysed benzylic C–H hydroxylation of trifluoromethylated arenes.
Metal-free synthesis of phosphinoylchroman-4-ones via a radical phosphinoylation–cyclization cascade mediated by K2S2O8
- Qiang Liu,
- Weibang Lu,
- Guanqun Xie and
- Xiaoxia Wang
Beilstein J. Org. Chem. 2020, 16, 1974–1982, doi:10.3762/bjoc.16.164
- sciences over the last few years [1][4][5]. In general, chroman-4-one derivatives could be obtained via a polarity reversal strategy enabled by the N-heterocyclic carbene (NHC)-catalyzed intramolecular Stetter reaction [6][7][8]. Besides, chroman-4-one derivatives were also constructed via intramolecular

Graphical Abstract

Figure 1: Biologically active compounds featuring the chroman-4-one framework.

Scheme 1: Methods to produce phosphonate-substituted chroman-4-ones.

Figure 2: X-ray structure of compound 3aa (CCDC 2002878).

Scheme 2: Scope of 2-(allyloxy)arylaldehydes. Reaction conditions: 1 (0.3 mmol, 1 equiv), 2a (1.5 equiv) [2f ...

Scheme 3: Scope of diphenylphosphine oxides. Reaction conditions: 1a (0.3 mmol, 1 equiv), 2 (1.5 equiv), DMSO...

Scheme 4: Gram-scale reaction.

Scheme 5: Control experiments and proposed mechanism.
Synergy between supported ionic liquid-like phases and immobilized palladium N-heterocyclic carbene–phosphine complexes for the Negishi reaction under flow conditions
- Edgar Peris,
- Raúl Porcar,
- María Macia,
- Jesús Alcázar,
- Eduardo García-Verdugo and
- Santiago V. Luis
Beilstein J. Org. Chem. 2020, 16, 1924–1935, doi:10.3762/bjoc.16.159
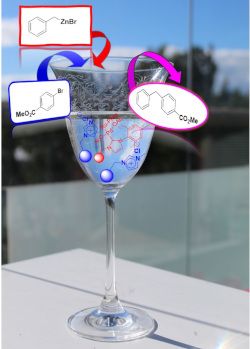

Graphical Abstract

Scheme 1: Synthesis of NHC-supported catalysts.

Scheme 2: Negishi benchmark reaction.

Figure 1: Negishi reaction catalyzed by immobilized NHC–Pd complexes. Conditions: methyl 4-bromobenzoate (0.2...

Scheme 3: Synthesis of immobilized NHC–Pd–RuPhos.

Figure 2: Negishi model reaction between 5 and 6 under flow conditions catalyzed by 4b. V = 0.535 mL, 363 mg ...

Figure 3: Negishi model reaction under flow conditions catalyzed by 8a. V = 2.9 mL, 1.25 g of catalyst, resid...

Figure 4: Negishi reaction between 5 and 6 catalyzed by 8a in the presence of SILLPs. a) Yield (%) vs time fo...

Figure 5: TEM images of the polymers after the Negishi reaction between 5 and 6. a) 8a, bar scale 20 nm, PdNP...

Scheme 4: Pd species immobilized onto SILLPs. i) 1 g SILLP 10, 100 mg PdCl2 in milli-Q® water (100 mL 1% HCl,...

Figure 6: Negishi reaction between 5 and 6 catalyzed by 11. 1 equiv methyl 4-bromobenzoate (6, 0.25 mmol), 2 ...

Figure 7: Negishi reaction between 5 and 6 under flow conditions catalyzed by 8a in the presence of a scaveng...

Figure 8: Effect of the structure of the SILLP scavenger for the Negishi reaction between 5 and 6 under flow ...

Figure 9: TEM images of the polymer after the Negishi reaction between 5 and 6 under flow conditions. a) 8a + ...
NHC-catalyzed enantioselective synthesis of β-trifluoromethyl-β-hydroxyamides
- Alyn T. Davies,
- Mark D. Greenhalgh,
- Alexandra M. Z. Slawin and
- Andrew D. Smith
Beilstein J. Org. Chem. 2020, 16, 1572–1578, doi:10.3762/bjoc.16.129
- Alyn T. Davies Mark D. Greenhalgh Alexandra M. Z. Slawin Andrew D. Smith EaStCHEM, School of Chemistry, University of St Andrews, North Haugh, St Andrews, Fife KY16 9ST, United Kingdom 10.3762/bjoc.16.129 Abstract The N-heterocyclic carbene (NHC)-catalyzed formal [2 + 2] cycloaddition between α
- moderate to good yield as a single diastereoisomer. Keywords: enantioselective catalysis; formal [2 + 2] cycloaddition; N-heterocyclic carbene; ring opening; trifluoromethyl group; Introduction The trifluoromethyl unit holds a prominent and privileged position within organic chemistry [1][2][3][4][5][6

Graphical Abstract

Figure 1: Organocatalytic enantioselective aldol approaches using trifluoroacetophenone derivatives.

Figure 2: NHC-catalyzed approaches to β-lactones using trifluoroacetophenone derivatives.

Scheme 1: Reaction scope with respect to the nucleophile. aIsolated yield of the product in >95:5 dr. bDeterm...

Scheme 2: Reaction scope with respect to the trifluoroacetophenone derivative and α-aroyloxyaldehyde. aIsolat...

Scheme 3: Proposed mechanism.
Disposable cartridge concept for the on-demand synthesis of turbo Grignards, Knochel–Hauser amides, and magnesium alkoxides
- Mateo Berton,
- Kevin Sheehan,
- Andrea Adamo and
- D. Tyler McQuade
Beilstein J. Org. Chem. 2020, 16, 1343–1356, doi:10.3762/bjoc.16.115

- combined with a solution-phase reagent, including: (1) copper(I) oxide to produce N-heterocyclic carbene–Cu(I) complexes for use as catalysts [13]; (2) proline to perform proline-based catalytic reactions [14]; (3) zinc powder to produce organozinc halides in tandem with Negishi couplings [15]; (4) zinc

Graphical Abstract

Figure 1: Comparing on-demand coffee and turbo Grignard pod-style machines.

Figure 2: Ranking of the 20 most cited Grignard reagents (SciFinder March 26, 2019).

Figure 3: On-demand prototype. A) Inside view of the pump with a flexible bag containing a yellow liquid layi...

Figure 4: Temperature evolution measured with thermocouples along the column outer surface at three different...

Figure 5: Stratified bicomponent column (Diba Omnifit EZ Solvent Plus) composed of magnesium (chips/powder, 1...

Scheme 1: Continuous flow synthesis of TMPMgCl⋅LiCl with a stratified packed-bed column of activated magnesiu...

Scheme 2: Continuous flow synthesis of TMPMgCl⋅LiBr with a stratified packed-bed column of activated magnesiu...

Scheme 3: Continuous flow synthesis of t-AmylOMgCl⋅LiCl with a stratified packed-bed column of activated magn...

Figure 6: Steady-state concentration stability during the conversion of iPrCl in THF (56 mL, 2.2 M) into iPrM...

Scheme 4: Synthesis of iPrMgCl⋅LiCl on the ODR prototype.

Scheme 5: Synthesis of HMDSMgCl⋅LiCl on the ODR prototype.
Recent advances in Cu-catalyzed C(sp3)–Si and C(sp3)–B bond formation
- Balaram S. Takale,
- Ruchita R. Thakore,
- Elham Etemadi-Davan and
- Bruce H. Lipshutz
Beilstein J. Org. Chem. 2020, 16, 691–737, doi:10.3762/bjoc.16.67

- undergoing proto-demetallation afforded the final product 46 (Scheme 11B). Initially, the reaction was limited to the generation of racemic products. Ultimately, optimization using McQuade’s six membered N-heterocyclic carbene (NHC) L2 [37] in combination with CuCl led to conditions applicable to different

Graphical Abstract

Scheme 1: Pharmaceuticals possessing a silicon or boron atom.

Scheme 2: The first Cu-catalyzed C(sp3)–Si bond formation.

Scheme 3: Conversion of benzylic phosphate 6 to the corresponding silane.

Scheme 4: Conversion of alkyl triflates to alkylsilanes.

Scheme 5: Conversion of secondary alkyl triflates to alkylsilanes.

Scheme 6: Conversion of alkyl iodides to alkylsilanes.

Scheme 7: Trapping of intermediate radical through cascade reaction.

Scheme 8: Radical pathway for conversion of alkyl iodides to alkylsilanes.

Scheme 9: Conversion of alkyl ester of N-hydroxyphthalimide to alkylsilanes.

Scheme 10: Conversion of gem-dibromides to bis-silylalkanes.

Scheme 11: Conversion of imines to α-silylated amines (A) and the reaction pathway (B).

Scheme 12: Conversion of N-tosylimines to α-silylated amines.

Scheme 13: Screening of diamine ligands.

Scheme 14: Conversion of N-tert-butylsulfonylimines to α-silylated amines.

Scheme 15: Conversion of aldimines to nonracemic α-silylated amines.

Scheme 16: Conversion of N-tosylimines to α-silylated amines.

Scheme 17: Reaction pathway [A] and conversion of aldehydes to α-silylated alcohols [B].

Scheme 18: Conversion of aldehydes to benzhydryl silyl ethers.

Scheme 19: Conversion of ketones to 1,2-diols (A) and conversion of imines to 1,2-amino alcohols (B).

Scheme 20: Ligand screening (A) and conversion of aldehydes to α-silylated alcohols (B).

Scheme 21: Conversion of aldehydes to α-silylated alcohols.

Scheme 22: 1,4-Additions to α,β-unsaturated ketones.

Scheme 23: 1,4-Additions to unsaturated ketones to give β-silylated derivatives.

Scheme 24: Additions onto α,β-unsaturated lactones to give β-silylated lactones.

Scheme 25: Conversion of α,β-unsaturated to β-silylated lactams.

Scheme 26: Conversion of N-arylacrylamides to silylated oxindoles.

Scheme 27: Conversion of α,β-unsaturated carbonyl compounds to silylated tert-butylperoxides.

Scheme 28: Catalytic cycle for Cu(I) catalyzed α,β-unsaturated compounds.

Scheme 29: Conversion of p-quinone methides to benzylic silanes.

Scheme 30: Conversion of α,β-unsaturated ketimines to regio- and stereocontrolled allylic silanes.

Scheme 31: Conversion of α,β-unsaturated ketimines to enantioenriched allylic silanes.

Scheme 32: Regioselective conversion of dienedioates to allylic silanes.

Scheme 33: Conversion of alkenyl-substituted azaarenes to β-silylated adducts.

Scheme 34: Conversion of conjugated benzoxazoles to enantioenriched β-silylated adducts.

Scheme 35: Conversion of α,β-unsaturated carbonyl indoles to α-silylated N-alkylated indoles.

Scheme 36: Conversion of β-amidoacrylates to α-aminosilanes.

Scheme 37: Conversion of α,β-unsaturated ketones to enantioenriched β-silylated ketones, nitriles, and nitro d...

Scheme 38: Regio-divergent silacarboxylation of allenes.

Scheme 39: Silylation of diazocarbonyl compounds, (A) asymmetric and (B) racemic.

Scheme 40: Enantioselective hydrosilylation of alkenes.

Scheme 41: Conversion of 3-acylindoles to indolino-silanes.

Scheme 42: Proposed mechanism for the silylation of 3-acylindoles.

Scheme 43: Silyation of N-chlorosulfonamides.

Scheme 44: Conversion of acyl silanes to α-silyl alcohols.

Scheme 45: Conversion of N-tosylaziridines to β-silylated N-tosylamines.

Scheme 46: Conversion of N-tosylaziridines to silylated N-tosylamines.

Scheme 47: Conversion of 3,3-disubstituted cyclopropenes to silylated cyclopropanes.

Scheme 48: Conversion of conjugated enynes to 1,3-bis(silyl)propenes.

Scheme 49: Proposed sequence for the Cu-catalyzed borylation of substituted alkenes.

Scheme 50: Cu-catalyzed synthesis of nonracemic allylic boronates.

Scheme 51: Cu–NHC catalyzed synthesis of α-substituted allylboronates.

Scheme 52: Synthesis of α-chiral (γ-alkoxyallyl)boronates.

Scheme 53: Cu-mediated formation of nonracemic cis- or trans- 2-substituted cyclopropylboronates.

Scheme 54: Cu-catalyzed synthesis of γ,γ-gem-difluoroallylboronates.

Scheme 55: Cu-catalyzed hydrofunctionalization of internal alkenes and vinylarenes.

Scheme 56: Cu-catalyzed Markovnikov and anti-Markovnikov borylation of alkenes.

Scheme 57: Cu-catalyzed borylation/ortho-cyanation/Cope rearrangement.

Scheme 58: Borylfluoromethylation of alkenes.

Scheme 59: Cu-catalyzed synthesis of tertiary nonracemic alcohols.

Scheme 60: Synthesis of densely functionalized and synthetically versatile 1,2- or 4,3-borocyanated 1,3-butadi...

Scheme 61: Cu-catalyzed trifunctionalization of allenes.

Scheme 62: Cu-catalyzed selective arylborylation of arenes.

Scheme 63: Asymmetric borylative coupling between styrenes and imines.

Scheme 64: Regio-divergent aminoboration of unactivated terminal alkenes.

Scheme 65: Cu-catalyzed 1,4-borylation of α,β-unsaturated ketones.

Scheme 66: Cu-catalyzed protodeboronation of α,β-unsaturated ketones.

Scheme 67: Cu-catalyzed β-borylation of α,β-unsaturated imines.

Scheme 68: Cu-catalyzed synthesis of β-trifluoroborato carbonyl compounds.

Scheme 69: Asymmetric 1,4-borylation of α,β-unsaturated carbonyl compounds.

Scheme 70: Cu-catalyzed ACB and ACA reactions of α,β-unsaturated 2-acyl-N-methylimidazoles.

Scheme 71: Cu-catalyzed diborylation of aldehydes.

Scheme 72: Umpolung pathway for chiral, nonracemic tertiary alcohol synthesis (top) and proposed mechanism for...

Scheme 73: Cu-catalyzed synthesis of α-hydroxyboronates.

Scheme 74: Cu-catalyzed borylation of ketones.

Scheme 75: Cu-catalyzed borylation of unactivated alkyl halides.

Scheme 76: Cu-catalyzed borylation of allylic difluorides.

Scheme 77: Cu-catalyzed borylation of cyclic and acyclic alkyl halides.

Scheme 78: Cu-catalyzed borylation of unactivated alkyl chlorides and bromides.

Scheme 79: Cu-catalyzed decarboxylative borylation of carboxylic acids.

Scheme 80: Cu-catalyzed borylation of benzylic, allylic, and propargylic alcohols.
A systematic review on silica-, carbon-, and magnetic materials-supported copper species as efficient heterogeneous nanocatalysts in “click” reactions
- Pezhman Shiri and
- Jasem Aboonajmi
Beilstein J. Org. Chem. 2020, 16, 551–586, doi:10.3762/bjoc.16.52
- Cu(I)-catalyzed cyclization between CRGO–N3 (93) and 1-propargyl-3-methylimidazolium bromide (94) was used to attach the imidazolium moiety to the surface of the CRGO. Finally, a graphene-containing copper complex of N-heterocyclic carbene, (NHC)-Cu (96), was produced via proton exchange of the
- -heterocyclic carbene) in multiple steps [28]. Initially, imidazole (28) was immobilized on the silica nanomaterial 27 by a nucleophilic attack of 28 on the iodopropylated silica nanomaterial 27 in acetonitrile under reflux for 48 h. The obtained 1-propylimidazole–nanosilica compound ImP–nSiO2 (29) was filtered
- thicker layers of Cu on Au nanoparticles. Other findings in this study were: a) copper leaching decreased using gold nanoparticles, b) increasing the dendron generation increased the diffusion resistance. As shown in Scheme 5, a silica nanomaterial was decorated with a novel copper complex of poly(N

Graphical Abstract

Scheme 1: Chemical structure of the catalysts 1a and 1b and their catalytic application in CuAAC reactions.

Scheme 2: Synthetic route to the catalyst 11 and its catalytic application in CuAAC reactions.

Scheme 3: Synthetic route of dendrons, illustrated using G2-AMP 23.

Scheme 4: The catalytic application of CuYAu–Gx-AAA–SBA-15 in a CuAAC reaction.

Scheme 5: Synthetic route to the catalyst 36.

Scheme 6: Application of the catalyst 36 in CuAAC reactions.

Scheme 7: The synthetic route to the catalyst 45 and catalytic application of 45 in “click” reactions.

Scheme 8: Synthetic route to the catalyst 48 and catalytic application of 48 in “click” reactions.

Scheme 9: Synthetic route to the catalyst 58 and catalytic application of 58 in “click” reactions.

Scheme 10: Synthetic route to the catalyst 64 and catalytic application of 64 in “click” reactions.

Scheme 11: Chemical structure of the catalyst 68 and catalytic application of 68 in “click” reactions.

Scheme 12: Chemical structure of the catalyst 69 and catalytic application of 69 in “click” reactions.

Scheme 13: Synthetic route to, and chemical structure of the catalyst 74.

Scheme 14: Application of the cayalyst 74 in “click” reactions.

Scheme 15: Synthetic route to, and chemical structure of the catalyst 78 and catalytic application of 78 in “c...

Scheme 16: Synthetic route to the catalyst 85.

Scheme 17: Application of the catalyst 85 in “click” reactions.

Scheme 18: Synthetic route to the catalyst 87 and catalytic application of 87 in “click” reactions.

Scheme 19: Chemical structure of the catalyst 88 and catalytic application of 88 in “click” reactions.

Scheme 20: Synthetic route to the catalyst 90 and catalytic application of 90 in “click” reactions.

Scheme 21: Synthetic route to the catalyst 96 and catalytic application of 96 in “click” reactions.

Scheme 22: Synthetic route to the catalyst 100 and catalytic application of 100 in “click” reactions.

Scheme 23: Synthetic route to the catalyst 102 and catalytic application of 23 in “click” reactions.

Scheme 24: Synthetic route to the catalysts 108–111.

Scheme 25: Catalytic application of 108–111 in “click” reactions.

Scheme 26: Synthetic route to the catalyst 121 and catalytic application of 121 in “click” reactions.

Scheme 27: Synthetic route to 125 and application of 125 in “click” reactions.

Scheme 28: Synthetic route to the catalyst 131 and catalytic application of 131 in “click” reactions.

Scheme 29: Synthetic route to the catalyst 136.

Scheme 30: Application of the catalyst 136 in “click” reactions.

Scheme 31: Synthetic route to the catalyst 141 and catalytic application of 141 in “click” reactions.

Scheme 32: Synthetic route to the catalyst 144 and catalytic application of 144 in “click” reactions.

Scheme 33: Synthetic route to the catalyst 149 and catalytic application of 149 in “click” reactions.

Scheme 34: Synthetic route to the catalyst 153 and catalytic application of 153 in “click” reactions.

Scheme 35: Synthetic route to the catalyst 155 and catalytic application of 155 in “click” reactions.

Scheme 36: Synthetic route to the catalyst 157 and catalytic application of 157 in “click” reactions.

Scheme 37: Synthetic route to the catalyst 162.

Scheme 38: Application of the catalyst 162 in “click” reactions.

Scheme 39: Synthetic route to the catalyst 167 and catalytic application of 167 in “click” reactions.

Scheme 40: Synthetic route to the catalyst 169 and catalytic application of 169 in “click” reactions.

Scheme 41: Synthetic route to the catalyst 172.

Scheme 42: Application of the catalyst 172 in “click” reactions.
Aerobic synthesis of N-sulfonylamidines mediated by N-heterocyclic carbene copper(I) catalysts
- Faïma Lazreg,
- Marie Vasseur,
- Alexandra M. Z. Slawin and
- Catherine S. J. Cazin
Beilstein J. Org. Chem. 2020, 16, 482–491, doi:10.3762/bjoc.16.43
- : catalysis; copper; copper catalysis; N-heterocyclic carbene; solvent-free; sulfonylamidines; Introduction Amide derivatives represent a ubiquitous molecular construct in chemistry [1][2][3]. This structural motif favours rearrangements that lead to high reactivity and associated bioactivity [4][5]. Indeed
- , NHCs (NHC = N-heterocyclic carbene) have become ligands of choice to permit the stabilisation and formation of highly reactive transition metal species [14]. Thus, significant advances have been achieved using this supporting ligand family [15][16][17][18][19]. Recently, our group contributed to this

Graphical Abstract

Scheme 1: Formation of sulfonyltriazoles and sulfonamidines.

Figure 1: Catalytic systems used in this study.

Scheme 2: Synthetic access to complexes 4–6 [30].

Scheme 3: Variation of sulfonylazides. Reaction conditions: phenylacetylene (0.5 mmol), sulfonyl azide (0.6 m...

Scheme 4: Variation of alkynes. Reaction conditions: alkyne (0.5 mmol), tosyl azide (0.6 mmol), diisopropylam...

Scheme 5: Variation of the amine substrate. Reaction conditions: phenylacetylene (0.5 mmol), tosyl azide (0.6...

Scheme 6: Reactivity of “non-sulfonyl” azide [33]. Reaction conditions: phenylacetylene (0.5 mmol), benzyl azide ...

Scheme 7: Reactivity of diphenylphosphoryl azide. Reaction conditions: phenylacetylene (0.5 mmol), diphenylph...

Scheme 8: Proposed mechanism for the formation of sulfonamidine.

Scheme 9: Stoichiometric reaction between 6 and 8.

Scheme 10: Synthesis of copper-acetylide intermediate A via [Cu(Cl)(Triaz)].

Scheme 11: Catalytic reaction involving copper-acetylide complex A.
Formal preparation of regioregular and alternating thiophene–thiophene copolymers bearing different substituents
- Atsunori Mori,
- Keisuke Fujita,
- Chihiro Kubota,
- Toyoko Suzuki,
- Kentaro Okano,
- Takuya Matsumoto,
- Takashi Nishino and
- Masaki Horie
Beilstein J. Org. Chem. 2020, 16, 317–324, doi:10.3762/bjoc.16.31
- by polymerization catalyzed by a nickel complex, leading to the regioregular HT polythiophene (Scheme 1) [8][9]. An additional feature of the deprotonative protocol for polythiophene is the possibility to use chlorothiophene 3, in which the use of a nickel N-heterocyclic carbene (NHC) complex was

Graphical Abstract

Scheme 1: Cross-coupling polymerization of thiophene.

Scheme 2: Polymerization of bithiophene.

Scheme 3: Preparation of chlorobithiophenes.

Scheme 4: Polymerization of chlorobithiophenes.

Figure 1: Solubility tests of alternating copolymer 6 (1 mg of material dissolved in 1 mL of the solvent).

Figure 2: XRD measurement and prediction of the bilayer lamellar structure of polymer 6c. a) XRD analysis. b)...
Allylic cross-coupling using aromatic aldehydes as α-alkoxyalkyl anions
- Akihiro Yuasa,
- Kazunori Nagao and
- Hirohisa Ohmiya
Beilstein J. Org. Chem. 2020, 16, 185–189, doi:10.3762/bjoc.16.21

- electrophile [8][10][11]. This paper describes in full detail the racemic system using allylic carbonates. The allylic cross-coupling of aromatic aldehydes and allylic carbonates with a silylboronate by the merging of a copper–N-heterocyclic carbene catalyst and a palladium–bisphosphine catalyst produced

Graphical Abstract

Scheme 1: Our strategy.

Scheme 2: Allylic cross-coupling using aldehydes as α-alkoxyalkyl anions.

Scheme 3: Substrate scope and reaction conditions. a) reactions were carried out with 1 (0.4 mmol), 2 (0.2 mm...

Scheme 4: Stoichiometric reaction.

Scheme 5: Possible pathway.
Recent advances in transition-metal-catalyzed incorporation of fluorine-containing groups
- Xiaowei Li,
- Xiaolin Shi,
- Xiangqian Li and
- Dayong Shi
Beilstein J. Org. Chem. 2019, 15, 2213–2270, doi:10.3762/bjoc.15.218
- fluorination of oxindoles with an axially chiral C2-symmetric N-heterocyclic carbene (NHC) palladium complex as a catalyst (Scheme 7a). The corresponding products were obtained in excellent yields but low to moderate enantioselectivities. Meanwhile, Wu and co-workers [42] developed a similar system using a

Graphical Abstract

Scheme 1: The main three strategies of fluorination: nucleophilic, electrophilic and radical fluorination.

Scheme 2: Doyle’s Pd-catalyzed fluorination of allylic chlorides.

Scheme 3: Allylic fluorination of 2- and 3-substituted propenyl esters.

Scheme 4: Regioselective allylic fluorination of cinnamyl phosphorothioate esters.

Scheme 5: Palladium-catalyzed aliphatic C–H fluorination reported by Doyle.

Scheme 6: Pd-catalyzed enantioselective fluorination of α-ketoesters followed by stereoselective reduction to...

Scheme 7: Pd-catalyzed C(sp3)–H fluorination of oxindoles.

Scheme 8: C–H fluorination of 8-methylquinoline derivatives with F− reagents.

Scheme 9: Fluorination of α-cyano acetates reported by van Leeuwen.

Scheme 10: The catalytic enantioselective electrophilic C–H fluorination of α-chloro-β-keto phosphonates.

Scheme 11: Fluorination of unactivated C(sp3)–H bonds directed by the bidentate PIP auxiliary.

Scheme 12: Fluorination of C(sp3)–H bonds at the β-position of carboxylic acids.

Scheme 13: Enantioselective benzylic C–H fluorination with a chiral transient directing group.

Scheme 14: Microwave-heated Pd-catalyzed fluorination of aryl alcohols.

Scheme 15: Fluorination of aryl potassium trifluoroborates.

Scheme 16: C(sp2)–F bond formation using precatalyst [L·Pd]2(cod).

Scheme 17: Pd-catalyzed fluorination of (hetero)aryl triflates and bromides.

Scheme 18: The Pd-catalyzed C–H fluorination of arenes with Selectfluor/NFSI.

Scheme 19: Pd(II)-catalyzed ortho-monofluorination protocol for benzoic acids.

Scheme 20: Pd-catalyzed C(sp2)–H bond fluorination of 2-arylbenzothiazoles.

Scheme 21: Nitrate-promoted fluorination of aromatic and olefinic C(sp2)–H bonds and proposed mechanism.

Scheme 22: Fluorination of oxalyl amide-protected benzylamine derivatives.

Scheme 23: C–H fluorination of benzaldehydes with orthanilic acids as transient directing group.

Scheme 24: Pd(II)-catalyzed aryl C–H fluorination with various directing groups.

Scheme 25: Cu-catalyzed aliphatic, allylic, and benzylic fluorination.

Scheme 26: Cu-catalyzed SN2 fluorination of primary and secondary alkyl bromides.

Scheme 27: Copper-catalyzed fluorination of alkyl triflates.

Scheme 28: Cu-catalyzed fluorination of allylic bromides and chlorides.

Scheme 29: Synthetic strategy for the fluorination of active methylene compounds.

Scheme 30: Fluorination of β-ketoesters using a tartrate-derived bidentate bisoxazoline-Cu(II) complex.

Scheme 31: Highly enantioselective fluorination of β-ketoesters and N-Boc-oxindoles.

Scheme 32: Amide group-assisted site-selective fluorination of α-bromocarbonyl compounds.

Scheme 33: Cu-mediated aryl fluorination reported by Sanford [77].

Scheme 34: Mono- or difluorination reactions of benzoic acid derivatives.

Scheme 35: Cu-catalyzed fluorination of diaryliodonium salts with KF.

Scheme 36: Copper(I)-catalyzed cross-coupling of 2-pyridylaryl bromides.

Scheme 37: AgNO3-catalyzed decarboxylative fluorination of aliphatic carboxylic acids.

Scheme 38: The Mn-catalyzed aliphatic and benzylic C–H fluorination.

Scheme 39: Iron(II)-promoted C–H fluorination of benzylic substrates.

Scheme 40: Ag-catalyzed fluorodecarboxylation of carboxylic acids.

Scheme 41: Vanadium-catalyzed C(sp3)–H fluorination.

Scheme 42: AgNO3-catalyzed radical deboronofluorination of alkylboronates and boronic acids.

Scheme 43: Selective heterobenzylic C–H fluorination with Selectfluor reported by Van Humbeck.

Scheme 44: Fe(II)-catalyzed site-selective fluorination guided by an alkoxyl radical.

Scheme 45: Fluorination of allylic trichloroacetimidates reported by Nguyen et al.

Scheme 46: Iridium-catalyzed fluorination of allylic carbonates with TBAF(t-BuOH)4.

Scheme 47: Iridium-catalyzed asymmetric fluorination of allylic trichloroacetimidates.

Scheme 48: Cobalt-catalyzed α-fluorination of β-ketoesters.

Scheme 49: Nickel-catalyzed α-fluorination of various α-chloro-β-ketoesters.

Scheme 50: Ni(II)-catalyzed enantioselective fluorination of oxindoles and β-ketoesters.

Scheme 51: Scandium(III)-catalyzed asymmetric C–H fluorination of unprotected 3-substituted oxindoles.

Scheme 52: Iron-catalyzed directed C–H fluorination.

Scheme 53: Electrophilic silver-catalyzed Ar–F bond-forming reaction from arylstannanes.

Figure 1: Nucleophilic, electrophilic and radical CF3 sources.

Scheme 54: Cu(I)-catalyzed allylic trifluoromethylation of unactivated terminal olefins.

Scheme 55: Direct copper-catalyzed trifluoromethylation of allylsilanes.

Scheme 56: Cupper-catalyzed enantioselective trifluoromethylation of five and six-membered ring β-ketoesters.

Scheme 57: Cu-catalyzed highly stereoselective trifluoromethylation of secondary propargyl sulfonates.

Scheme 58: Remote C(sp3)–H trifluoromethylation of carboxamides and sulfonamides.

Scheme 59: Trifluoromethylation of allylsilanes with photoredox catalysis.

Scheme 60: Ag-catalyzed decarboxylative trifluoromethylation of aliphatic carboxylic acids in aqueous CH3CN.

Scheme 61: Decarboxylative trifluoromethylation of aliphatic carboxylic acids via combined photoredox and copp...

Scheme 62: Palladium-catalyzed Ar–CF3 bond-forming reaction.

Scheme 63: Palladium-catalyzed trifluoromethylation of arenes with diverse heterocyclic directing groups.

Scheme 64: Pd-catalyzed trifluoromethylation of indoles as reported by Liu.

Scheme 65: Pd-catalyzed trifluoromethylation of vinyl triflates and vinyl nonaflates.

Scheme 66: Pd(II)-catalyzed ortho-trifluoromethylation of aromatic C–H bonds.

Scheme 67: Visible-light-induced Pd(OAc)2-catalyzed ortho-trifluoromethylation of acetanilides with CF3SO2Na.

Scheme 68: CuI-catalyzed trifluoromethylation of aryl- and alkenylboronic acids.

Scheme 69: Cu-catalyzed trifluoromethylation of aryl- and vinylboronic acids.

Scheme 70: Copper-catalyzed trifluoromethylation of α,β-unsaturated carboxylic acids.

Scheme 71: Formation of C(sp2)–CF3 bond catalyzed by copper(I) complex.

Scheme 72: Loh’s Cu(I)-catalyzed trifluoromethylation of enamides and electron-deficient alkenes.

Scheme 73: Copper and iron-catalyzed decarboxylative tri- and difluoromethylation.

Scheme 74: Cu-catalyzed trifluoromethylation of hydrazones developed by Bouyssi.

Scheme 75: Cu(I)-catalyzed trifluoromethylation of terminal alkenes.

Scheme 76: Cu/Ag-catalyzed decarboxylative trifluoromethylation of cinnamic acids.

Scheme 77: Copper-catalyzed direct alkenyl C–H trifluoromethylation.

Scheme 78: Copper(I/II)-catalyzed direct trifluoromethylation of styrene derivatives.

Scheme 79: Regioselective trifluoromethylation of pivalamido arenes and heteroarenes.

Scheme 80: Synthesis of trifluoromethylquinones in the presence of copper(I).

Scheme 81: Oxidative trifluoromethylation of imidazoheterocycles in ionic liquid/water.

Scheme 82: A mild and fast continuous-flow trifluoromethylation of coumarins using a CuI/CF3SO2Na/TBHP system.

Scheme 83: Copper-catalyzed oxidative trifluoromethylation of various 8-aminoquinolines.

Scheme 84: PA-directed copper-catalyzed trifluoromethylation of anilines.

Scheme 85: Trifluoromethylation of potassium vinyltrifluoroborates catalyzed by Fe(II).

Scheme 86: Alkenyl trifluoromethylation catalyzed by Ru(phen)3Cl2 as photocatalyst.

Scheme 87: Ru-catalyzed trifluoromethylation of alkenes by Akita’s group.

Scheme 88: Ir-catalyzed Cvinyl–CF3 bond formation of α,β-unsaturated carboxylic acids.

Scheme 89: Ag(I)-catalyzed denitrative trifluoromethylation of β-nitrostyrenes.

Scheme 90: Photocatalyzed direct trifluoromethylation of aryl and heteroaryl C–H bonds.

Scheme 91: Rhenium (MTO)-catalyzed direct trifluoromethylation of aromatic substrates.

Scheme 92: Trifluoromethylation of unprotected anilines under [Ir(ppy)3] catalyst.

Scheme 93: Oxidative trifluoromethylation of imidazopyridines and imidazoheterocycles.

Scheme 94: Ruthenium-catalyzed trifluoromethylation of (hetero)arenes with trifluoroacetic anhydride.

Scheme 95: Phosphovanadomolybdic acid-catalyzed direct C–H trifluoromethylation.

Scheme 96: Picolinamide-assisted ortho-trifluoromethylation of arylamines.

Scheme 97: A nickel-catalyzed C–H trifluoromethylation of free anilines.

Scheme 98: Cu-mediated trifluoromethylation of terminal alkynes reported by Qing.

Scheme 99: Huang’s C(sp)–H trifluoromethylation using Togni’s reagent.

Scheme 100: Cu-catalyzed methods for trifluoromethylation with Umemoto’s reagent.

Scheme 101: The synthesis of alkynyl-CF3 compounds in the presence of fac-[Ir(ppy)3] under visible-light irradi...

Scheme 102: Pd-catalyzed Heck reaction reported by Reutrakul.

Scheme 103: Difluoromethylation of enamides and ene-carbamates.

Scheme 104: Difluoromethylation of α,β-unsaturated carboxylic acids.

Scheme 105: Copper-catalyzed direct C(sp2)–H difluoroacetylation reported by Pannecoucke and co-workers.

Scheme 106: Difluoroalkylation of aldehyde-derived hydrazones with functionalized difluoromethyl bromides.

Scheme 107: Photoredox-catalyzed C–H difluoroalkylation of aldehyde-derived hydrazones.

Scheme 108: Synergistic ruthenium(II)-catalyzed C–H difluoromethylation reported by Ackermann.

Scheme 109: Visible-light photocatalytic decarboxylation of α,β-unsaturated carboxylic acids.

Scheme 110: Synthesis of difluorinated ketones via S-alkyl dithiocarbamates obtained from acyl chlorides and po...

Scheme 111: Synthesis of aryl and heteroaryl difluoromethylated phosphonates.

Scheme 112: Difluoroalkylation of secondary propargyl sulfonates using Cu as the catalyst.

Scheme 113: Ru(II)-mediated para-selective difluoromethylation of anilides and their derivatives.

Scheme 114: Bulky diamine ligand promoted cross-coupling of difluoroalkyl bromides.

Scheme 115: Copper-catalyzed C3–H difluoroacetylation of quinoxalinones.

Scheme 116: Copper(I) chloride-catalyzed trifluoromethylthiolation of enamines, indoles and β-ketoesters.

Scheme 117: Copper-boxmi-catalyzed asymmetric trifluoromethylthiolation of β-ketoesters.

Scheme 118: Direct Cu-catalyzed trifluoromethylthiolation of boronic acids and alkynes.

Scheme 119: Cu-catalyzed synthesis of α-trifluoromethylthio-substituted ketones.

Scheme 120: Trifluoromethylthiolation reactions promoted by diazotriflone and copper.

Scheme 121: Halide activation of N-(trifluoromethylthio)phthalimide.

Scheme 122: The visible light-promoted trifluoromethylthiolation reported by Glorius.

Scheme 123: Synthesis of α-trifluoromethylthioesters via Goossen’s approach.

Scheme 124: Photoinduced trifluoromethylthiolation of diazonium salts.

Scheme 125: Ag-mediated trifluoromethoxylation of aryl stannanes and arylboronic acids.

Scheme 126: Catalytic (hetero)aryl C–H trifluoromethoxylation under visible light.

Scheme 127: Photoinduced C–H-bond trifluromethoxylation of (hetero)arenes.
1,2,3-Triazolium macrocycles in supramolecular chemistry
- Mastaneh Safarnejad Shad,
- Pulikkal Veettil Santhini and
- Wim Dehaen
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- mechanically interlocked molecules and molecular machines. We will not concern ourselves in this review with the applications of metal complexes based on N-heterocyclic carbene coordination chemistry, derived of triazoliums or with applications as ionic liquids [26][27][28]. 2. Anion recognition Due to the

Graphical Abstract

Figure 1: Hydrogen, halogen or chalcogen bonding to anions within a bistriazolium macrocycle.

Figure 2: Main synthetic strategies towards macrocyclic triazoliums.

Figure 3: Chemical structure of compound 1 (1a, 1b and 1c) and 2.

Figure 4: Chemical structure of compound 3 and 4.

Figure 5: Chemical structure of compound 5.

Figure 6: Chemical structure of compound 6.

Figure 7: Chemical structure of compound 7.

Figure 8: Chemical structure of compound 8.

Figure 9: Chemical structures of compound 9.

Figure 10: Chemical structures of compound 10, 11 and 12.

Figure 11: Chemical structure of compound 13.

Figure 12: Chemical structure of compound 15 including the sigma-connected TCNQ dimer.

Figure 13: Chemical structure of compound 16 for the kinetic resolution of epoxides.

Figure 14: Chemical structure of compound 17a (bisnaphtho crown ether shown).

Figure 15: Jump rope in molecular double-lasso compounds 18.

Figure 16: Chemical structure of compound 19 and acid–base triggered motions.
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
- Iwona E. Głowacka,
- Aleksandra Trocha,
- Andrzej E. Wróblewski and
- Dorota G. Piotrowska
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- -hydroxyester 163 was produced from the epoxide 162 employing N-heterocyclic carbene catalysis. Openings of the aziridine ring in 163a with azide or in 163b with in acetic acid provided enantiomerically pure methyl (3S,4S)-4,5-di-N-Boc-amino-3-hydroxypentanoate 164 or 4-N-Boc-amino-3,5-dihydroxypentanoate 165

Graphical Abstract

Figure 1: Examples of three-carbon chirons.

Figure 2: Structures of derivatives of N-(1-phenylethyl)aziridine-2-carboxylic acid 5–8.

Figure 3: Synthetic equivalency of aziridine aldehydes 6.

Scheme 1: Synthesis of N-(1-phenylethyl)aziridine-2-carboxylates 5. Reagents and conditions: a) TEA, toluene,...

Scheme 2: Absolute configuration at C2 in (2S,1'S)-5a. Reagents and conditions: a) 20% HClO4, 80 °C, 30 h the...

Scheme 3: Major synthetic strategies for a 2-ketoaziridine scaffold [R* = (R)- or (S)-1-phenylethyl; R′ = Alk...

Scheme 4: Synthesis of cyanide (2S,1'S)-13. Reagents and conditions: a) NH3, EtOH/H2O, rt, 72 h; b) Ph3P, CCl4...

Scheme 5: Synthesis of key intermediates (R)-16 and (R)-17 for (R,R)-formoterol (14) and (R)-tamsulosin (15)....

Scheme 6: Synthesis of mitotic kinesin inhibitors (2R/S,1'R)-23. Reagents and conditions: a) H2, Pd(OH)2, EtO...

Scheme 7: Synthesis of (R)-mexiletine ((R)-24). Reagents and conditions: a) TsCl, TEA, DMAP, CH2Cl2, rt, 1 h;...

Scheme 8: Synthesis of (−)-cathinone ((S)-27). Reagents and conditions: a) PhMgBr, ether, 0 °C; b) H2, 10% Pd...

Scheme 9: Synthesis of N-Boc-norpseudoephedrine ((1S,2S)-(+)-29) and N-Boc-norephedrine ((1R,2S)-29). Reagent...

Scheme 10: Synthesis of (−)-ephedrine ((1R,2S)-31). Reagents and conditions: a) TfOMe, MeCN then NaBH3CN, rt; ...

Scheme 11: Synthesis of xestoaminol C ((2S,3R)-35), 3-epi-xestoaminol C ((2S,3S)-35) and N-Boc-spisulosine ((2S...

Scheme 12: Synthesis of ʟ-tryptophanol ((S)-41). Reagents and conditions: a) CDI, MeCN, rt, 1 h then TMSI, MeC...

Scheme 13: Synthesis of ʟ-homophenylalaninol ((S)-42). Reagents and conditions: a) NaH, THF, 0 °C to −78 °C, 1...

Scheme 14: Synthesis of ᴅ-homo(4-octylphenyl)alaninol ((R)-47) and a sphingolipid analogue (R)-48. Reagents an...

Scheme 15: Synthesis of florfenicol ((1R,2S)-49). Reagents and conditions: a) (S)-1-phenylethylamine, TEA, MeO...

Scheme 16: Synthesis of natural tyroscherin ((2S,3R,6E,8R,10R)-55). Reagents and conditions: a) I(CH2)3OTIPS, t...

Scheme 17: Syntheses of (−)-hygrine (S)-61, (−)-hygroline (2S,2'S)-62 and (−)-pseudohygroline (2S,2'R)-62. Rea...

Scheme 18: Synthesis of pyrrolidine (3S,3'R)-68, a fragment of the fluoroquinolone antibiotic PF-00951966. Rea...

Scheme 19: Synthesis of sphingolipid analogues (R)-76. Reagents and conditions: a) BnBr, Mg, THF, reflux, 6 h;...

Scheme 20: Synthesis of ᴅ-threo-PDMP (1R,2R)-81. Reagents and conditions: a) TMSCl, NaI, MeCN, rt, 1 h 50 min,...

Scheme 21: Synthesis of the sphingolipid analogue SG-14 (2S,3S)-84. Reagents and conditions: a) LiAlH4, THF, 0...

Scheme 22: Synthesis of the sphingolipid analogue SG-12 (2S,3R)-88. Reagents and conditions: a) 1-(bromomethyl...

Scheme 23: Synthesis of sphingosine-1-phosphate analogues DS-SG-44 and DS-SG-45 (2S,3R)-89a and (2S,3R)-89a. R...

Scheme 24: Synthesis of N-Boc-safingol ((2S,3S)-95) and N-Boc-ᴅ-erythro-sphinganine ((2S,3R)-95). Reagents and...

Scheme 25: Synthesis of ceramide analogues (2S,3R)-96. Reagents and conditions: a) NaBH4, ZnCl2, MeOH, −78 °C,...

Scheme 26: Synthesis of orthogonally protected serinols, (S)-101 and (R)-102. Reagents and conditions: a) BnBr...

Scheme 27: Synthesis of N-acetyl-3-phenylserinol ((1R,2R)-105). Reagents and conditions: a) AcOH, CH2Cl2, refl...

Scheme 28: Synthesis of (S)-linezolid (S)-107. Reagents and conditions: a) LiAlH4, THF, 0 °C to reflux; b) Boc2...

Scheme 29: Synthesis of (2S,3S,4R)-2-aminooctadecane-1,3,4-triol (ᴅ-ribo-phytosphingosine) (2S,3S,4R)-110. Rea...

Scheme 30: Syntheses of ᴅ-phenylalanine (R)-116. Reagents and conditions: a) AcOH, CH2Cl2, reflux, 4 h; b) MsC...

Scheme 31: Synthesis of N-Boc-ᴅ-3,3-diphenylalanine ((R)-122). Reagents and conditions: a) PhMgBr, THF, −78 °C...

Scheme 32: Synthesis of ethyl N,N’-di-Boc-ʟ-2,3-diaminopropanoate ((S)-125). Reagents and conditions: a) NaN3,...

Scheme 33: Synthesis of the bicyclic amino acid (S)-(+)-127. Reagents and conditions: a) BF3·OEt2, THF, 60 °C,...

Scheme 34: Synthesis of lacosamide, (R)-2-acetamido-N-benzyl-3-methoxypropanamide (R)-130. Reagents and condit...

Scheme 35: Synthesis of N-Boc-norfuranomycin ((2S,2'R)-133). Reagents and conditions: a) H2C=CHCH2I, NaH, THF,...

Scheme 36: Synthesis of MeBmt (2S,3R,4R,6E)-139. Reagents and conditions: a) diisopropyl (S,S)-tartrate (E)-cr...

Scheme 37: Synthesis of (+)-polyoxamic acid (2S,3S,4S)-144. Reagents and conditions: a) AD-mix-α, MeSO2NH2, t-...

Scheme 38: Synthesis of the protected 3-hydroxy-ʟ-glutamic acid (2S,3R)-148. Reagents and conditions: a) LiHMD...

Scheme 39: Synthesis of (+)-isoserine (R)-152. Reagents and conditions: a) AcCl, MeCN, rt, 0.5 h then Na2CO3, ...

Scheme 40: Synthesis of (3R,4S)-N3-Boc-3,4-diaminopentanoic acid (3R,4S)-155. Reagents and conditions: a) Ph3P...

Scheme 41: Synthesis of methyl (2S,3S,4S)-4-(dimethylamino)-2,3-dihydroxy-5-methoxypentanoate (2S,3S,4S)-159. ...

Scheme 42: Syntheses of methyl (3S,4S) 4,5-di-N-Boc-amino-3-hydroxypentanoate ((3S,4S)-164), methyl (3S,4S)-4-N...

Scheme 43: Syntheses of (3R,5S)-5-(aminomethyl)-3-(4-methoxyphenyl)dihydrofuran-2(3H)-one ((3R,5S)-168). Reage...

Scheme 44: Syntheses of a series of imidazolin-2-one dipeptides 175–177 (for R' and R'' see text). Reagents an...

Scheme 45: Syntheses of (2S,3S)-N-Boc-3-hydroxy-2-hydroxymethylpyrrolidine ((2S,3S)-179). Reagents and conditi...

Scheme 46: Syntheses of enantiomers of 1,4-dideoxy-1,4-imino-ʟ- and -ᴅ-lyxitols (2S,3R,4S)-182 and (2R,3S,4R)-...

Scheme 47: Synthesis of 1,4-dideoxy-1,4-imino-ʟ-ribitol (2S,3S,4R)-182. Reagents and conditions: a) AcOH, CH2Cl...

Scheme 48: Syntheses of 1,4-dideoxy-1,4-imino-ᴅ-arabinitol (2R,3R,4R)-182 and 1,4-dideoxy-1,4-imino-ᴅ-xylitol ...

Scheme 49: Syntheses of natural 2,5-imino-2,5,6-trideoxy-ʟ-gulo-heptitol ((2S,3R,4R,5R)-184) and its C4 epimer...

Scheme 50: Syntheses of (−)-dihydropinidine ((2S,6R)-187a) (R = C3H7) and (2S,6R)-isosolenopsins (2S,6R)-187b ...

Scheme 51: Syntheses of (+)-deoxocassine ((2S,3S,6R)-190a, R = C12H25) and (+)-spectaline ((2S,3S,6R)-190b, R ...

Scheme 52: Synthesis of (−)-microgrewiapine A ((2S,3R,6S)-194a) and (+)-microcosamine A ((2S,3R,6S)-194b). Rea...

Scheme 53: Syntheses of ʟ-1-deoxynojirimycin ((2S,3S,4S,5R)-200), ʟ-1-deoxymannojirimycin ((2S,3S,4S,5S)-200) ...

Scheme 54: Syntheses of 1-deoxy-ᴅ-galacto-homonojirimycin (2R,3S,4R,5S)-211. Reagents and conditions: a) MeONH...

Scheme 55: Syntheses of 7a-epi-hyacinthacine A1 (1S,2R,3R,7aS)-220. Reagents and conditions: a) TfOTBDMS, 2,6-...

Scheme 56: Syntheses of 8-deoxyhyacinthacine A1 ((1S,2R,3R,7aR)-221). Reagents and conditions: a) H2, Pd/C, PT...

Scheme 57: Syntheses of (+)-lentiginosine ((1S,2S,8aS)-227). Reagents and conditions: a) (EtO)2P(O)CH2COOEt, L...

Scheme 58: Syntheses of 8-epi-swainsonine (1S,2R,8S,8aR)-231. Reagents and conditions: a) Ph3P=CHCOOMe, MeOH, ...

Scheme 59: Synthesis of a protected vinylpiperidine (2S,3R)-237, a key intermediate in the synthesis of (−)-sw...

Scheme 60: Synthesis of a modified carbapenem 245. Reagents and conditions: a) AcOEt, LiHMDS, THF, −78 °C, 1.5...






